Aerobic oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic acid over Au/Hydrotalcite catalyst − role of support and synthesis methodology on the activity and stability
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
The oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) is key in producing bio-based plastics like polyethylene furanoate (PEF), a sustainable alternative to petrochemical materials. This work reports a systematic study on how the hydrotalcite (HT) support precursors—specifically using Na+ or NH4+ precursors and the choice of synthesis method (deposition–precipitation, DP, or sol immobilization, SI) influence the catalytic performance and stability of Au/HT catalysts under base-free reaction conditions. Au/HTNa DP achieved 100 % HMF conversion and FDCA yield without an external base. This high activity is reflected in its turnover frequency (TOF), reaching 12.1 h−1 per basic site and 287.4 h−1 per gold site. The superior performance of Au/HTNa DP is attributed to the strong synergy between gold nanoparticles (AuNPs) and weak basic sites (OH– groups) of the HT, whose abundance is dictated by the choice of precursor. In contrast, Au/HTNH4 SI, despite higher Au dispersion, showed lower activity due to reduced basicity. Magnesium leaching was identified as the primary cause of catalyst deactivation, and a regeneration strategy employing Mg(OH)2 was developed to successfully restore both the structure and basicity of the catalyst. These findings offer practical insights into the design of recyclable, base-free catalytic systems for sustainable FDCA production.
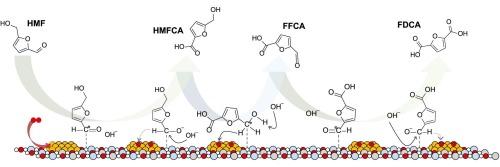
金/水滑石催化剂上5-羟甲基糠醛好氧氧化制2,5-呋喃二羧酸——载体和合成方法对活性和稳定性的影响
5-羟甲基糠醛(HMF)氧化为2,5-呋喃二羧酸(FDCA)是生产聚呋喃酸酯(PEF)等生物基塑料的关键,聚呋喃酸酯是石化材料的可持续替代品。本文系统地研究了水滑石(HT)在无碱反应条件下如何支持前驱体(特别是使用Na+或NH4+前驱体)和合成方法(沉积-沉淀,DP或溶胶固定化,SI)的选择对Au/HT催化剂的催化性能和稳定性的影响。Au/HTNa DP在没有外部碱的情况下实现了100%的HMF转化率和FDCA收率。这种高活性反映在其周转率(TOF)上,每个碱基位点达到12.1 h−1,每个金位点达到287.4 h−1。Au/HTNa DP的优异性能归因于金纳米颗粒(AuNPs)与HT的弱碱性位点(OH -基团)之间的强协同作用,其丰度取决于前驱体的选择。相比之下,Au/HTNH4 SI虽然具有较高的Au分散性,但由于碱度降低,活性较低。镁浸出是催化剂失活的主要原因,采用Mg(OH)2再生策略成功地恢复了催化剂的结构和碱度。这些发现为设计可回收的、无碱的催化体系以实现FDCA的可持续生产提供了实际的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


