Mouse radial spoke 3 is a metabolic and regulatory hub in cilia
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
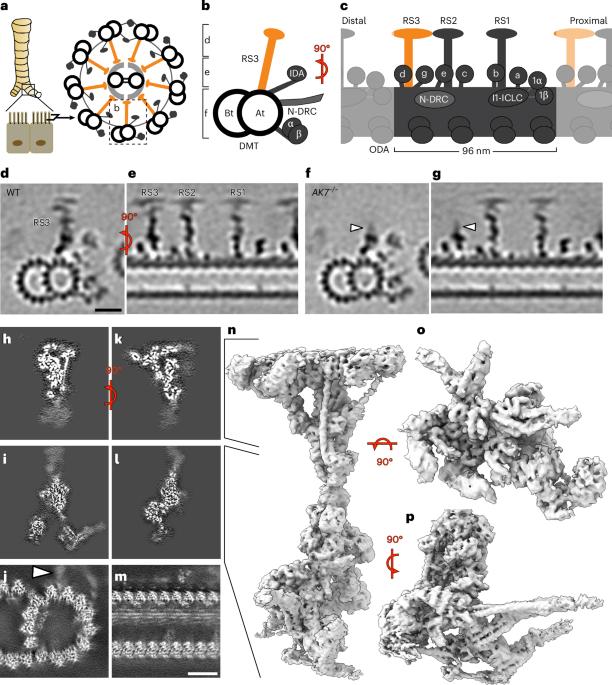
Cilia are microtubule-based organelles that have important roles in cell sensing, signaling and motility. Recent studies have revealed the atomic structures of many multicomponent ciliary complexes, elucidating their mechanisms of action. However, little is known about the structure, proteome and function of full-length radial spoke 3 (RS3), a conserved complex that transmits mechanochemical signals to coordinate ciliary motility. Here, we combined single-particle cryo-electron microscopy, cryo-electron tomography, proteomic analysis and computational modeling to determine the three-dimensional structure and atomic model of RS3 from mouse respiratory cilia. We reveal all RS3 components, including regulatory and metabolic enzymes such as a protein kinase A subunit, adenylate kinases (AKs) and malate dehydrogenases. Furthermore, we confirm RS3 loss in AK7-deficient mice, which exhibit motility defects. Our findings identify RS3 as an important regulatory and metabolic hub that maintains sufficient adenosine triphosphate for sustained ciliary beating, providing insights into the etiology of ciliopathies. Zhao et al. uncover the protein composition and atomic structure of ciliary radial spoke 3, revealing it as a metabolic hub that regulates ciliary motility, providing insights into ciliary diseases
小鼠径向辐条3是纤毛的代谢和调节中枢
纤毛是基于微管的细胞器,在细胞感知、信号传导和运动中起重要作用。近年来的研究揭示了许多多组分纤毛配合物的原子结构,阐明了它们的作用机制。然而,对于全长径向辐条3 (RS3)的结构、蛋白质组学和功能知之甚少,RS3是一个保守的复合物,传递机械化学信号以协调纤毛运动。本研究结合单粒子低温电子显微镜、低温电子断层扫描、蛋白质组学分析和计算模型,确定了小鼠呼吸纤毛RS3的三维结构和原子模型。我们揭示了所有RS3成分,包括调节和代谢酶,如蛋白激酶a亚基,腺苷酸激酶(AKs)和苹果酸脱氢酶。此外,我们证实了ak7缺陷小鼠的RS3缺失,这些小鼠表现出运动缺陷。我们的研究结果确定RS3是一个重要的调节和代谢中心,维持足够的三磷酸腺苷维持纤毛跳动,为纤毛病的病因学提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


