Roles of multiple TEF30-associated intermediate complexes in the repair and reassembly of photosystem II in Chlamydomonas reinhardtii
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
During oxygenic photosynthesis, photosystem II (PSII) uses light energy for oxidizing water and reducing plastoquinone. It is susceptible to photodamage, and the damaged PSII is repaired through a sophisticated biological process assisted by numerous auxiliary proteins. Here we report the cryogenic electron microscopy structures of four PSII-repair complexes from Chlamydomonas reinhardtii associated with the Thylakoid Enriched Fraction 30 (TEF30, an orthologue of plant MET1) protein—namely, a TEF30–PSII core monomer (TEF30-C), two types of TEF30–PSII core dimers (types I and II, TEF302-C2-I and TEF302-C2-II) and a TEF30-C2S-type PSII–LHCII supercomplex (TEF30-C2S; S, strongly associated light-harvesting complex II trimer). TEF30 mediates the assembly of CP43 with the RC47 module by clamping on the stromal surfaces and prevents the premature association of peripheral antennae with PSII-C. In the transition from TEF302-C2-I to TEF302-C2-II, TEF30-C2S and mature C2S2, one PSII core slides along the dimerization interface against the adjacent one by 22–35 Å, generating a zigzagged surface for accommodating the peripheral antennae. These results suggest that the PSII repair process undergoes multiple TEF30-mediated intermediate states to form intact PSII–LHCII supercomplexes. Cryo-EM structures of four TEF30-associated photosystem II repair complexes from Chlamydomonas reinhardtii reveal versatile roles of TEF30 in the reassembly of the photosystem II core monomer, dimer and supercomplex through multiple intermediate states.
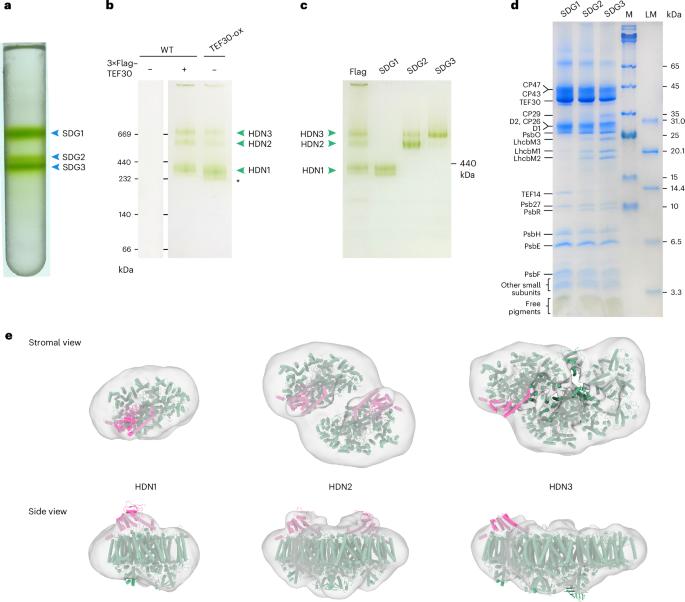
多个tef30相关中间复合物在莱茵衣藻光系统II修复和重组中的作用
在含氧光合作用中,光系统II (PSII)利用光能氧化水并还原质体醌。PSII易受光损伤,受损的PSII通过许多辅助蛋白辅助的复杂生物过程进行修复。本文报道了莱因衣藻与类囊体富集部分30 (TEF30,植物MET1的同源物)蛋白相关的四种psii修复复合物的低温电镜结构,即TEF30- psii核心单体(TEF30- c),两种TEF30- psii核心二聚体(I型和II型,TEF302-C2-I和TEF302-C2-II)和TEF30- c2s型PSII-LHCII超复合物(TEF30- c2s;S,强关联的光收集配合物II三聚体)。TEF30通过夹紧基质表面介导CP43与RC47模块的组装,并防止外周天线与PSII-C的过早关联。在从TEF302-C2-I过渡到TEF302-C2-II、TEF30-C2S和成熟的C2S2过程中,一个PSII核心沿着二聚化界面与相邻的PSII核心滑动22-35 Å,形成一个锯齿形表面,用于容纳周边天线。这些结果表明,PSII修复过程经历了多个tef30介导的中间状态,形成完整的PSII - lhcii超复合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


