Pharmacokinetics of Orally Administered Phenazopyridine in Goats With Obstructive Urolithiasis
Abstract
Background
Phenazopyridine is used for ancillary pain management in the treatment of goats with obstructive urolithiasis. However, there are no published studies on the pharmacokinetics, safety, or efficacy of phenazopyridine in goats.
Hypothesis/Objectives
Determine the pharmacokinetic parameters of phenazopyridine after oral administration in goats with obstructive urolithiasis after tube cystostomy surgery.
Animals
Six male goats, ages 3 months to 4 years.
Methods
Prospective, observational study. Goats presenting to a veterinary institution diagnosed with obstructive urolithiasis underwent a tube cystostomy surgery. After surgery, phenazopyridine (4 mg/kg PO q12h) was administered. Plasma and urine samples were collected at predetermined intervals, and the concentration of phenazopyridine and clinically relevant metabolites was determined using ultra high-performance liquid chromatography with mass spectrometry. The pharmacokinetic parameters were determined using non-compartmental analysis.
Results
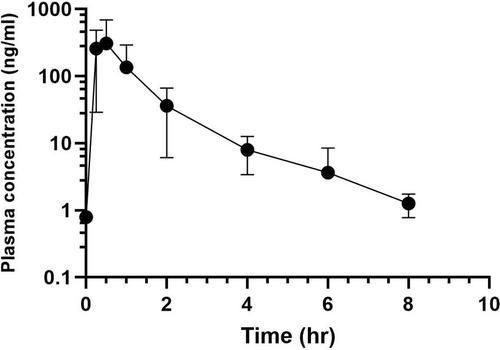
The harmonic mean terminal elimination plasma half-life (T1/2), geometric mean maximum plasma concentration (Cmax), and area under the curve (AUC) were 0.5 h (0.22–1.57 h), 263.4 ng/mL (137.35–1047.88 ng/mL), and 0.69 h*ng/mL (0.10–2.99 h*ng/mL), respectively for phenazopyridine. The concentration of phenazopyridine in urine samples was below the limit of assay detection (1.5 ng/mL) in all but one sample.
Conclusions and Clinical Importance
Phenazopyridine was rapidly eliminated from plasma and did not concentrate at detectable levels in the urine after oral administration.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


