Catalytic system-controlled divergent reactions of pyrazolidinones with 3-alkynyl-3-hydroxyisoindolinones to construct diversified nitrogen-containing heterocyclic scaffolds†
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A catalytic system-controlled divergent reaction was reported to construct three distinct nitrogen-containing heterocycles from readily available starting materials via a precise chemical bond activation and annulation cascade. Notably, this is the first capture of pyrazolidinones and propargyl alcohols to construct tetrahydropyrimidinones via selective N–N bond activation and to generate previously unreported 3-acylindoles. This protocol demonstrates a broad substrate scope, moderate to good yields, and valuable transformations, laying a robust foundation for drug discovery applications.
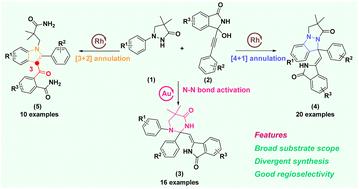
催化系统控制的吡唑烷酮与3-炔基-3-羟基异吲哚酮的发散反应构建多样化含氮杂环支架
报道了一个催化系统控制的发散反应,通过精确的化学键激活和环化级联,从现成的原料中构建了三个不同的含氮杂环。值得注意的是,这是第一次捕获吡唑烷酮和丙炔醇通过选择性N-N键激活来构建四氢嘧啶酮,并生成以前未报道的3-酰基吲哚。该方案展示了广泛的底物范围,中等到良好的产量,和有价值的转化,为药物发现应用奠定了坚实的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


