SEL1L–HRD1-mediated ERAD in mammals
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
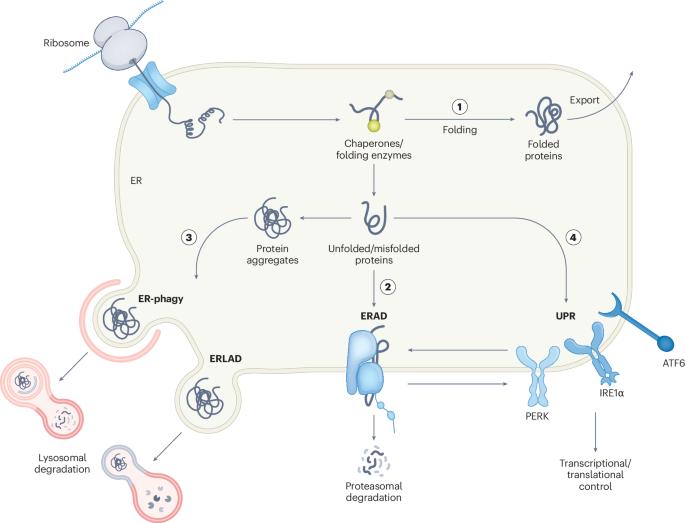
Endoplasmic reticulum-associated degradation (ERAD) is a critical quality control mechanism responsible for eliminating misfolded or unassembled proteins. It maintains endoplasmic reticulum homeostasis, ensures a proper folding environment and regulates substrate protein levels. Following its discovery in the late 1980s and early 1990s, research on ERAD in mammals—particularly that mediated by the conserved protein complex comprising suppressor/enhancer of Lin-12-like protein 1-like (SEL1L) and HMG-CoA reductase degradation protein 1 (HRD1)—has advanced substantially over the past decade. SEL1L–HRD1-mediated ERAD is now recognized as a fundamental process in mammals that governs various physiological functions largely in a substrate-specific manner. In humans, mutations in this complex have been causally linked to ERAD-associated neurodevelopmental disorders with onset in infancy (ENDI) and ENDI-agammaglobulinaemia. This Review highlights the SEL1L–HRD1-mediated ERAD pathway, exploring its machinery, molecular mechanism and physiological relevance and potential therapeutic strategies targeting this system. This Review discusses emerging insights into SEL1L–HRD1-mediated endoplasmic reticulum-associated degradation, including the underlying molecular mechanisms, physiological relevance for human disease and potential therapeutic opportunities.
sel1l - hrd1介导的哺乳动物ERAD
内质网相关降解(ERAD)是一种关键的质量控制机制,负责消除错误折叠或未组装的蛋白质。它维持内质网稳态,确保适当的折叠环境并调节底物蛋白水平。自20世纪80年代末和90年代初发现ERAD以来,对哺乳动物ERAD的研究,特别是由包含lin -12样蛋白1样(SEL1L)和HMG-CoA还原酶降解蛋白1 (HRD1)抑制/增强子的保守蛋白复合物介导的研究,在过去十年中取得了实质性进展。sel1l - hrd1介导的ERAD现在被认为是哺乳动物的一个基本过程,主要以底物特异性的方式控制各种生理功能。在人类中,这种复合物的突变与erd相关的婴儿期起病神经发育障碍(ENDI)和ENDI-无球蛋白血症有因果关系。本文重点介绍了sel1l - hrd1介导的ERAD通路,探讨了其机制、分子机制、生理相关性以及针对该系统的潜在治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


