Multimodal in vitro characterization techniques for assessing the adhesion of transdermal patches: A proof-of-concept study
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-21
DOI:10.1016/j.ejpb.2025.114783
引用次数: 0
Abstract
Adhesion is a critical factor in determining the quality, efficacy, and safety of transdermal patches. The adhesive properties directly influence drug permeation and flux, especially for matrix system patches. For potent therapeutics, the strength of adhesion and any minor tampering during wear can significantly impact the patch’s in vivo performance. Adhesive properties can vary over time due to changes in polymer interactions, with storage-induced three-dimensional alterations potentially affecting both adhesive strength and drug characteristics. Despite these complexities, no standardized method currently exists for measuring adhesive strength in transdermal patches. Therefore, reliable and consistent testing methods are essential to ensure patch quality and therapeutic efficacy. This study presents a multimodal approach to assess the adhesion of transdermal patches, incorporating traditional probe-tack and in vitro permeation tests, along with innovative techniques such as interferometry for surface topography analysis and infrared thermography to evaluate structural and adhesive deficiencies. The feasibility of these methods was validated by tampering fresh and expired patches across multiple rounds (2R, 5R, 10R). Validation was confirmed by measuring patch thickness before and after tampering with digital calipers, quantifying removed drug, and analyzing patch roughness and topography via interferometry. Adhesive performance was further assessed using the probe-tack test, while nicotine permeation profiles (both intact and tampered) were evaluated using vertical Franz diffusion cells. Statistical analysis, including one-way ANOVA and Tukey’s post-hoc test, identified significant differences between the datasets. The study utilized two different brands of 14 mg nicotine patches as models. Results indicated that the reference patches exhibited superior adhesive performance and uniform distribution compared to the test patches. The data demonstrates the potential of thermal imaging and interferometry as complementary techniques to distinguish adhesive performance differences in Q2-equivalent transdermal patches produced by different manufacturers.
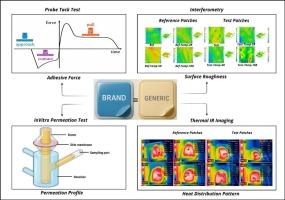
评估透皮贴片粘附性的多模式体外表征技术:一项概念验证研究。
粘附性是决定透皮贴剂质量、疗效和安全性的关键因素。黏附性能直接影响药物的渗透和通量,特别是对于基质系统贴片。对于有效的治疗方法,粘附强度和磨损过程中的任何轻微篡改都会显著影响贴片的体内性能。由于聚合物相互作用的变化,粘附性能会随着时间的推移而变化,储存引起的三维变化可能会影响粘附强度和药物特性。尽管有这些复杂性,目前还没有标准化的方法来测量透皮贴片的粘附强度。因此,可靠和一致的测试方法对于保证贴片质量和治疗效果至关重要。本研究提出了一种多模式的方法来评估透皮贴片的粘附性,结合传统的探针粘连和体外渗透试验,以及创新的技术,如干涉测量法的表面形貌分析和红外热成像,以评估结构和粘合缺陷。通过跨多轮(2R, 5R, 10R)篡改新鲜和过期补丁,验证了这些方法的可行性。通过数字卡尺测量篡改前后的贴片厚度,定量去除的药物,并通过干涉测量分析贴片的粗糙度和地形,证实了有效性。使用探针-粘接试验进一步评估粘附性能,同时使用垂直Franz扩散池评估尼古丁渗透曲线(完整和篡改)。统计分析,包括单因素方差分析和Tukey事后检验,确定了数据集之间的显著差异。本研究采用两种不同品牌的14个 mg尼古丁贴片作为模型。结果表明,与试验贴片相比,参考贴片具有更好的粘接性能和均匀分布。数据表明,热成像和干涉测量作为互补技术的潜力,可以区分不同制造商生产的q2等效透皮贴片的粘附性能差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


