Gene dosage-dependent roles of Mkx in postnatal tendon development and maintenance revealed by conditional deletion
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
The transcription factor Mohawk (Mkx) contributes to tendon development and differentiation, as demonstrated by conventional knockout studies. However, the temporal requirements and gene dosage effects of Mkx in postnatal tendon maturation and maintenance remain unclear. To address these questions, we generated a novel conditional knockout mouse model harboring a loxP-flanked allele and a Venus-CreERT2 knock-in allele at the Mkx locus by crossing Mkx Venus-CreERT2/+ with Mkx flox/+ lines. Tamoxifen was administered at two distinct stages: early postnatal (P3) and adult (6-week-old). Mkx Venus-CreERT2/+ mice exhibited mild reductions in tendon thickness and alterations in collagen fibril organization, while conditional deletion of Mkx (Mkx Venus-CreERT2/flox with tamoxifen induction) resulted in more pronounced defects. Time-course analysis revealed that both early postnatal and adult Mkx deletion led to progressive changes in tendon morphology, with TEM analysis showing a tendency toward reduced collagen fibril diameters. RNA-seq revealed distinct transcriptional changes associated with ECM organization and tendon homeostasis in Mkx-deficient tendons. These findings reveal previously unrecognized gene dosage effects of Mkx and suggest that maintaining appropriate levels of Mkx may be critical for tendon homeostasis, providing new insights into tendon biology and potential therapeutic strategies for tendon-related pathologies.
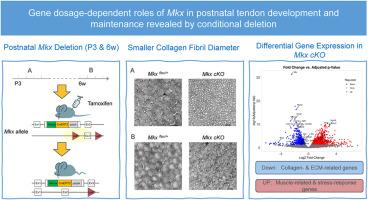
条件缺失揭示了Mkx在出生后肌腱发育和维持中的基因剂量依赖性作用。
转录因子Mohawk (Mkx)有助于肌腱的发育和分化,正如常规敲除研究所证明的那样。然而,Mkx在出生后肌腱成熟和维持中的时间需求和基因剂量效应尚不清楚。为了解决这些问题,我们通过将MkxVenus-CreERT2/+与Mkxflox/+杂交,建立了一种新的条件敲除小鼠模型,该模型在Mkx位点含有一个loxp -侧翼等位基因和一个Venus-CreERT2敲入等位基因。他莫昔芬在两个不同的阶段给药:产后早期(P3)和成年(6周大)。MkxVenus-CreERT2/+小鼠表现出肌腱厚度轻度减少和胶原纤维组织改变,而Mkx的条件缺失(MkxVenus-CreERT2/flox与他莫昔芬诱导)导致更明显的缺陷。时间过程分析显示,出生后早期和成年后Mkx缺失都会导致肌腱形态的进行性变化,TEM分析显示胶原纤维直径减少的趋势。RNA-seq揭示了mkx缺陷肌腱中与ECM组织和肌腱稳态相关的明显转录变化。这些发现揭示了先前未被认识到的Mkx基因剂量效应,并表明维持适当水平的Mkx可能对肌腱稳态至关重要,为肌腱生物学和肌腱相关疾病的潜在治疗策略提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


