The iolinid mite Pronematus ubiquitus controls a key tomato pest and pathogen by both predation and induction of specific plant defenses
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Tomato production is persistently challenged by pests such as the tomato russet mite (Aculops lycopersici) and pathogens like tomato powdery mildew (Oidium neolycopersici). Traditionally managed with chemical pesticides, sustainable alternatives are needed. This study evaluates the dual role of the omnivorous predatory mite, Pronematus ubiquitus, in directly suppressing pest populations and pathogen infection and indirectly inducing plant defense responses in tomato. Laboratory and greenhouse experiments were conducted using the standard cultivar Castlemart and its jasmonate-deficient mutant, defenseless-1 (def-1), to disentangle the contributions of direct predation from plant-mediated defenses. Pre-exposure of tomato plants to P. ubiquitus significantly reduced A. lycopersici oviposition on Castlemart but not on def-1 plants, implicating jasmonic acid (JA)-dependent defenses in mediating this effect. In vitro assays further demonstrated that P. ubiquitus feeding delayed spore germination and slowed down the development of powdery mildew. Under greenhouse conditions, prolonged exposure to high densities of P. ubiquitus resulted in a marked reduction in powdery mildew incidence compared to both untreated controls and plants treated with the established defense inducer, Macrolophus pygmaeus. Transcriptomic analyses revealed that infestation by P. ubiquitus triggered extensive reprogramming of defense-related gene expression, including the upregulation of key components involved in JA, salicylic acid, and ethylene signaling pathways, as well as genes associated with secondary metabolite biosynthesis and pathogen recognition. Collectively, these findings demonstrate that P. ubiquitus confers enhanced protection against both A. lycopersici and O. neolycopersici through a combination of direct predation and the elicitation of multifaceted plant defenses, offering promising implications for sustainable pest management in tomato cultivation.
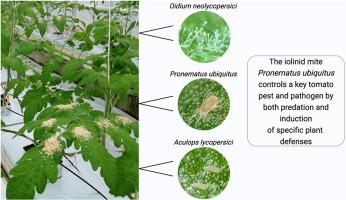
烟碱螨(proematus ubiquitus)通过捕食和诱导特定的植物防御来控制一种重要的番茄害虫和病原体。
番茄生产一直受到番茄赤褐色螨(aclops lycopersici)等害虫和番茄白粉病(Oidium neolycopersici)等病原体的挑战。传统上使用化学农药进行管理,需要可持续的替代品。本研究评价了杂食性掠食性螨proematus ubiquitus在番茄中直接抑制害虫种群和病原菌侵染以及间接诱导植物防御反应的双重作用。利用标准品种Castlemart及其茉莉素缺乏突变体defless -1 (def-1)进行了室内和温室试验,以阐明植物介导的防御对直接捕食的贡献。预暴露番茄植株可显著降低茄红霉在Castlemart植株上的产卵量,但对def1植株无显著影响,提示茉莉酸(jasmonic acid, JA)依赖性防御机制参与了这一作用。体外实验进一步证明,泛菌取食可延缓白粉病孢子萌发,减缓白粉病的发展。在温室条件下,与未经处理的对照和用已建立的防御诱导剂pygmaeus处理的植株相比,长时间暴露于高密度的泛白霉导致白粉病发病率显著降低。转录组学分析显示,泛藻侵染引发了防御相关基因表达的广泛重编程,包括JA、水杨酸和乙烯信号通路的关键成分,以及与次生代谢物生物合成和病原体识别相关的基因的上调。综上所述,这些研究结果表明,通过直接捕食和诱导多方面的植物防御相结合,泛叶番茄对番茄红霉和新番茄红霉具有增强的保护作用,为番茄种植的可持续害虫管理提供了有希望的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


