Structure engineering for organic ammonium ion batteries: A controllable distribution of Cu atoms in Mn-based Prussian blue analogues
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Ammonium ion batteries (AIBs) attract extensive attention owing to the abundant resources, eco-friendly and high safety. Multivalent Prussian blue analogues (PBAs), characterized by multi-electron transfer and high operating voltage, are considered as promising cathodes for AIBs. However, their structural stability is compromised by irreversible structural evolution and metal ion dissolution. Herein, a structure engineering strategy is proposed to boost NH4+ ion storage by regulating the distribution of Cu atoms in Mn-based PBAs. Cu atoms not only enrich on the surface to form a uniform layer but are doped inside the Mn-based PBAs. The resulting PBAs (noted as CuxMn1-xHCF) with an optimal x value of 0.15, deliver a stable capacity of 110.1 mAh g−1 at 50 mA g−1 and an exceptional capacity retention of 91 % over 4000 cycles at 200 mA g−1. Ex-situ characterizations and density functional theory (DFT) calculations reveal that the controllable distribution of Cu atoms significantly enhances the structural stability and suppresses the dissolution of Mn atoms. Notably, this strategy can be extended to other PBAs materials. We believe that this work advances the development of high-performance cathode materials for AIBs.
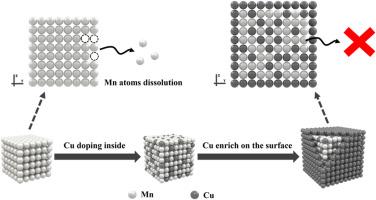
有机铵离子电池的结构工程:锰基普鲁士蓝类似物中Cu原子的可控分布
铵离子电池因其资源丰富、环保、安全性高等特点而受到广泛关注。多价普鲁士蓝类似物(PBAs)具有多电子转移和高工作电压的特点,被认为是一种很有前途的aib阴极。然而,不可逆的结构演化和金属离子的溶解破坏了它们的结构稳定性。本文提出了一种结构工程策略,通过调节锰基PBAs中Cu原子的分布来提高NH4+离子的存储。Cu原子不仅在表面富集形成均匀层,而且在mn基PBAs中掺杂。所得到的PBAs(称为CuxMn1-xHCF)的最佳x值为0.15,在50 mA g - 1下提供110.1 mAh g - 1的稳定容量,在200 mA g - 1下提供超过4000次循环91%的卓越容量保持。非原位表征和密度泛函理论(DFT)计算表明,Cu原子的可控分布显著提高了结构稳定性,抑制了Mn原子的溶解。值得注意的是,该策略可以扩展到其他PBAs材料。我们相信这项工作促进了高性能aib正极材料的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


