Metabolomic insights into the prebiotic and metabolic regulatory properties of ellagic acid and urolithins on probiotic-like bacteria in vitro
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
Abstract
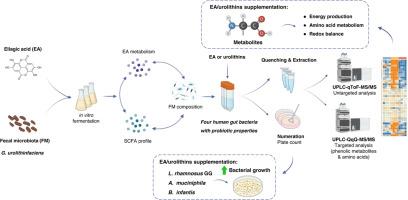
Mounting evidence suggests that dietary polyphenols exert health benefits partly through their favorable interactions with gut bacteria. However, little is known about polyphenol’s metabolic regulatory effects towards individual bacteria at the molecular level. Ellagic acid (EA), a polyphenol abundantly present in plant-based foods, was found to exhibit prebiotic properties through differential interactions with probiotic-like bacteria, including the EA-to-urolithin converting species such as Gordonibacter urolithinfaciens (G. uro). This study aimed to investigate the crosstalk between EA and EA-responsive beneficial bacteria, including both conventional and next-generation probiotics originating from the human gut, and to understand the underlying mechanism by which EA exerts prebiotic activities in vitro. The influence of EA and urolithins on probiotic bacteria was investigated at the levels of fecal microbiota and individual strains via anaerobic culturomics and metabolomics approaches. Results indicate that dietary-level EA favorably regulated gut microbial composition through the enrichment of probiotic genera (e.g., Bifidobacterium and Akkermansia) in vitro. Regarding individual bacteria, EA supplementation promoted the growth of Lacticaseibacillus rhamnosus GG and Bifidobacterium infantis. Integrated targeted and untargeted metabolomic analyses of intracellular and extracellular metabolites revealed that EA/urolithins modulated metabolic pathways associated with amino acid metabolism, energy production and oxidative stress. Furthermore, G. uro exhibited strong EA uptake ability, facilitating the urolithin bioconversion and cellular accumulation in a dose-dependent manner. Overall, this study provides in-depth understanding on how dietary polyphenols with prebiotic properties regulate the growth and metabolic functions of probiotic-like bacteria.
代谢组学研究鞣花酸和尿石素对体外益生菌样细菌的益生元和代谢调节特性
越来越多的证据表明,饮食中的多酚对健康的好处部分是通过它们与肠道细菌的有利相互作用来实现的。然而,多酚在分子水平上对单个细菌的代谢调节作用知之甚少。鞣花酸(EA)是一种富含植物性食物的多酚,研究发现,鞣花酸(EA)通过与益生菌样细菌的不同相互作用,表现出益生元的特性,包括将EA转化为尿素的物种,如gordonibacterurothinfaciens (G. uro)。本研究旨在探讨EA与对EA有反应的有益菌(包括源自人类肠道的传统益生菌和下一代益生菌)之间的串扰,并了解EA在体外发挥益生元活性的潜在机制。通过厌氧培养组学和代谢组学方法,研究了EA和尿石素对粪菌群和个别菌株的益生菌的影响。结果表明,日粮水平的EA通过富集益生菌属(如双歧杆菌和Akkermansia),有利于调节肠道微生物组成。在单个细菌方面,添加EA促进了鼠李糖乳杆菌GG和婴儿双歧杆菌的生长。细胞内和细胞外代谢物的综合靶向和非靶向代谢组学分析显示,EA/尿石素调节与氨基酸代谢、能量产生和氧化应激相关的代谢途径。此外,G. uro表现出较强的EA摄取能力,促进尿素的生物转化和细胞积累,并呈剂量依赖性。综上所述,本研究深入了解了具有益生元特性的膳食多酚如何调节益生菌样细菌的生长和代谢功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


