Photoenzymatic stereoablative enantioconvergence of γ-chiral oximes via hydrogen atom transfer
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Producing enantioenriched molecules from racemic mixtures is essential for manufacturing. Traditional methods such as resolution, deracemization and enantioconvergent catalysis primarily involve separating or converting enantiomers without altering their structures, or functionalization of stereocentres at or proximal to functional groups. However, there are challenges in enantioselectively forging C–H bonds that are remote from functional groups via hydrogen atom transfer (HAT) with these methods. Here we introduce a strategy for the photoenzymatic stereoablative enantioconvergence of γ-chiral oximes using repurposed flavin-dependent ene-reductases. A photoinduced single-electron reduction of the γ-chiral oxime by an ene-reductase generates an iminyl radical, which then undergoes stereoablative 1,5-HAT at the γ-stereocentre. Subsequent chiral reconstruction through enzymatic HAT and spontaneous imine hydrolysis yields the γ-chiral ketone with high enantioselectivity. This work provides a robust method for remote stereoablative enantioconvergent HAT and broadens the synthetic utility of photobiocatalysis. Remote C–H bond formation via photoenzymatic hydrogen atom transfer has enabled the precise installation of remote stereocentres but is still in its infancy. Here, the authors report the photoenzymatic stereoablative enantioconvergence of γ-chiral oximes using repurposed flavin-dependent ene-reductases.
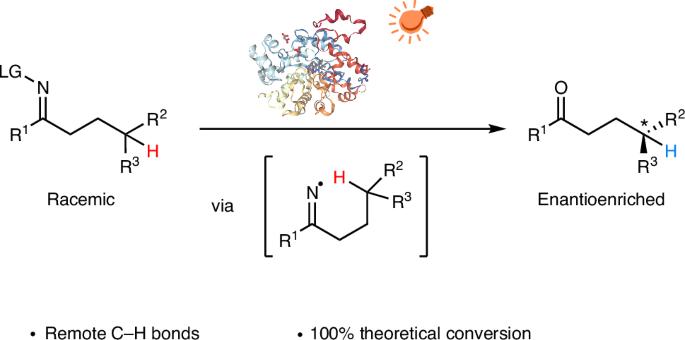
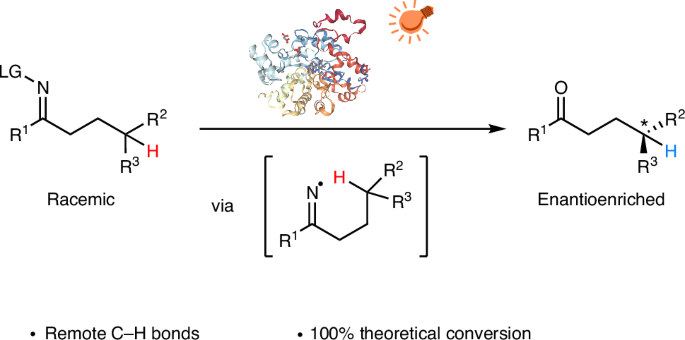
通过氢原子转移的γ-手性肟的光酶立体蚀性对映收敛
从外消旋混合物中生产富集对映体分子是制造的必要条件。传统的方法,如拆分、去消旋和对映聚合催化,主要涉及分离或转化对映体而不改变其结构,或在官能团上或邻近的立体中心进行功能化。然而,用这些方法通过氢原子转移(HAT)对映选择性地锻造远离官能团的碳氢键存在挑战。在这里,我们介绍了一种利用黄素依赖的烯还原酶进行γ-手性肟光酶立体消融对映收敛的策略。由烯还原酶光诱导的γ-手性肟的单电子还原产生亚胺基自由基,然后在γ-立体中心发生1,5- hat立体蚀变。随后通过酶促HAT和自发亚胺水解进行手性重建,得到具有高对映选择性的γ-手性酮。这项工作为远程立体蚀对映会聚HAT提供了一种可靠的方法,并拓宽了光生物催化的合成用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


