Organophotoredox-catalyzed deconstructive alkylation/Truce–Smiles rearrangement cascade involving spiro-dihydroquinazolinones and activated alkenes†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An organophotoredox-catalyzed cascade reaction involving C–C σ-bond cleavage, C–C bond formation, and a subsequent Truce–Smiles rearrangement has been developed using spiro-dihydroquinazolinones and activated alkenes. This aromaticity-driven deconstructive functionalization strategy efficiently yields valuable quinazolinone-containing long-chain amides bearing an α-all-carbon quaternary center. Mild redox-neutral conditions, good functional group compatibility and high atom economy are the noteworthy features of this methodology.
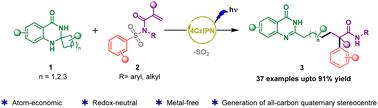
涉及螺-二氢喹唑啉酮和活化烯烃的有机光氧化还原催化解构烷基化/和平-微笑重排级联
利用螺-二氢喹啉酮和活化烯烃,建立了一种有机光氧化还原催化的级联反应,包括C-C σ键裂解、C-C键形成和随后的Truce-Smiles重排。这种芳香驱动的解构功能化策略有效地产生了具有α-全碳季中心的含喹唑啉酮长链酰胺。温和的氧化还原中性条件、良好的官能团相容性和高原子经济性是该方法的显著特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


