Acute high-altitude hypoxia induced NLRP3 inflammasome activation in pulmonary artery smooth muscle cells by BMAL1 targeting mitochondrial VDAC1-mediated MtDNA leakage
Abstract
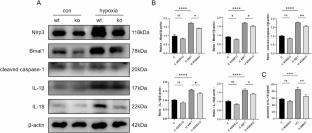
Hypoxia-induced inflammatory injury is an important pathological mechanism underlying the progression of acute mountain sickness (AMS). Recent studies reported that molecular clock could control mitochondrial pathways to involve hypoxic and inflammatory responses. Excessively released mitochondrial DNA (mtDNA) acts as a damage-associated molecular pattern (DAMP) to trigger inflammation in many diseases. Herein, we subjected mice at a simulated altitude of 5500 m for 3 days and found that the expression levels of inflammatory cytokines were significantly increased in mouse pulmonary arteries, accompanied by mtDNA release and NLRP3 inflammasome activation in the pulmonary artery smooth muscle cells (PASMCs). RNA-sequencing and loss- and gain-of function experiments indicated that the core clock component BMAL1 regulated mtDNA leakage in PASMCs, and smooth muscle-specific Bmal1 knockout significantly alleviated the pulmonary arterial inflammation under acute high-altitude hypoxia. Mechanically, BMAL1 as a transcription factor directly promoted the transcriptional expression of Voltage-dependent anion channel 1 (VDAC1) and exacerbated the VDAC1-mediated mtDNA leakage under hypoxia, which activated NLRP3 inflammasome signaling in PASMCs and induced vascular inflammation. Our work provides mechanistic insights into the hypoxia-induced inflammation in PASMCs and may provide a novel therapeutic approaching for targeting BMAL1-VDAC1 in AMS.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


