Sorption-induced metal isotope fractionation: Two decades of advances in methodologies, mechanisms, and kinetics
IF 10
1区 地球科学
Q1 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
Abstract
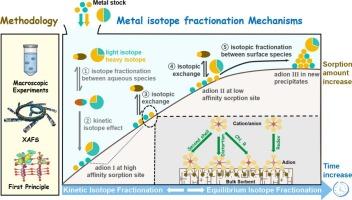
Stable metal isotopes are widely applied as tracers to address the complex biogeochemical cycling of metallic elements, attracting attention from diverse scientific disciplines, including geology, oceanography, biology, archaeology, and environmental sciences. In the low-temperature systems of Earth's surface environment, significant fractionations of metal isotopes occur during sorption processes at solid-water interfaces, which influence variations of metal isotope compositions on Earth's surface. Over the past two decades, advanced techniques, especially X-ray absorption fine spectroscopy (XAFS) and ab initio quantum chemistry, have been applied to elucidate the mechanisms of isotope fractionation, greatly enhancing our understanding of these phenomena. This review summarized the molecular-level mechanisms underlying metal isotope fractionation induced by sorption, leveraging insights from XAFS and ab initio quantum chemical studies. It emphasizes the crucial role of relative mass difference in determining the overall isotopic fractionation across various metals. Coordination chemistry emerges as a primary determinant for this fractionation, with the valence state playing an important role, particularly for redox-sensitive metals. Temperature occasionally impacts these processes and warrants consideration. While some sorption-induced isotopic fractionation exhibits kinetic effects, the underlying mechanisms, including kinetic isotope effects due to diffusion and the evolution of sorption mechanisms, remain poorly understood. These advancements promise to improve our ability to predict isotopic variations on Earth's surface more accurately and to further the application of metal isotopes in low-temperature systems.
吸附诱导的金属同位素分馏:方法、机制和动力学的二十年进展
稳定金属同位素作为示踪剂被广泛应用于研究金属元素复杂的生物地球化学循环,引起了地质学、海洋学、生物学、考古学和环境科学等学科的广泛关注。在地球表面环境的低温系统中,金属同位素在固水界面的吸附过程中发生了显著的分馏,这影响了地球表面金属同位素组成的变化。近二十年来,以x射线吸收精细光谱(XAFS)和从头算量子化学为代表的先进技术已被应用于阐明同位素分馏机理,极大地提高了我们对这些现象的认识。本文利用XAFS和从头算量子化学研究的见解,总结了吸附诱导金属同位素分馏的分子水平机制。它强调了相对质量差在确定各种金属的总体同位素分馏中的关键作用。配位化学成为这种分馏的主要决定因素,价态起着重要作用,特别是对氧化还原敏感的金属。温度偶尔会影响这些过程,值得考虑。虽然一些吸附诱导的同位素分馏表现出动力学效应,但其潜在的机制,包括由扩散引起的动力学同位素效应和吸附机制的演化,仍然知之甚少。这些进展有望提高我们更准确地预测地球表面同位素变化的能力,并进一步推动金属同位素在低温系统中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Earth-Science Reviews
地学-地球科学综合
CiteScore
21.70
自引率
5.80%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
Covering a much wider field than the usual specialist journals, Earth Science Reviews publishes review articles dealing with all aspects of Earth Sciences, and is an important vehicle for allowing readers to see their particular interest related to the Earth Sciences as a whole.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


