Improved serum biomarker detection via self-clickable Cu2O nanoparticle-coated Ag slides with oriented and stable antibody presentation on antifouling surfaces
IF 10.5
1区 生物学
Q1 BIOPHYSICS
引用次数: 0
Abstract
We propose an innovative approach to immobilize antibodies (Abs) on solid supports, addressing challenges in generating immunosensor arrays. This method involves a self-clickable Cu2O-nanoparticle-coated silver (Cu2O@Ag) surface for boronic acid (BA) functionalization, facilitating the irreversible immobilization of native Abs. The surface catalyzes azide-alkyne cycloaddition with azido-containing BA molecules without requiring an external Cu(I) ion source. Simultaneously, a metal-enhanced fluorescence effect amplifies the array detection signal, enabling ultrasensitive serum biomarker measurement. This process improves Ab orientation, maximizing antigen-binding site exposure for target protein interaction. The Cu2O@Ag glass slides show enhanced sensitivity, reduced nonspecific adsorption, and detection limits of 0.15, 0.07, 1.70, and 0.24 ng/mL for CRP, SAP, SAA, and PSA detection, respectively, which are below the diagnostic thresholds. When validated in a multiplex format with human serum samples, the assay demonstrated strong specificity and sensitivity in detecting key cancer biomarkers (CRP, SAP, SAA, AFP, CEA, and PSA). This Cu2O@Ag strategy for nonengineered Ab immobilization has broad implications for improving affinity-based protein detection assays.
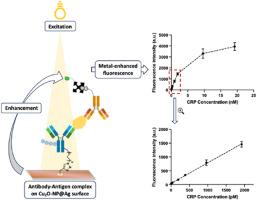
改进血清生物标志物检测,通过自点击Cu2O纳米粒子包被银载玻片定向和稳定的抗体在防污表面
我们提出了一种创新的方法将抗体(Abs)固定在固体载体上,解决了产生免疫传感器阵列的挑战。该方法采用可自点击的cu20纳米颗粒包覆银(Cu2O@Ag)表面,用于硼酸(BA)的功能化,促进天然抗体的不可逆固定。该表面催化叠氮-炔环加成与含叠氮的BA分子,而不需要外部Cu(I)离子源。同时,金属增强的荧光效应放大了阵列检测信号,使超灵敏的血清生物标志物测量成为可能。这一过程改善了Ab取向,最大化抗原结合位点暴露靶蛋白相互作用。Cu2O@Ag玻片检测CRP、SAP、SAA和PSA的灵敏度提高,非特异性吸附减少,检出限分别为0.15、0.07、1.70和0.24 ng/mL,均低于诊断阈值。当与人类血清样本进行多重验证时,该分析在检测关键癌症生物标志物(CRP, SAP, SAA, AFP, CEA和PSA)方面表现出很强的特异性和敏感性。这种Cu2O@Ag非工程Ab固定策略对改善基于亲和力的蛋白质检测分析具有广泛的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biosensors and Bioelectronics
工程技术-电化学
CiteScore
20.80
自引率
7.10%
发文量
1006
审稿时长
29 days
期刊介绍:
Biosensors & Bioelectronics, along with its open access companion journal Biosensors & Bioelectronics: X, is the leading international publication in the field of biosensors and bioelectronics. It covers research, design, development, and application of biosensors, which are analytical devices incorporating biological materials with physicochemical transducers. These devices, including sensors, DNA chips, electronic noses, and lab-on-a-chip, produce digital signals proportional to specific analytes. Examples include immunosensors and enzyme-based biosensors, applied in various fields such as medicine, environmental monitoring, and food industry. The journal also focuses on molecular and supramolecular structures for enhancing device performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


