A sandwich-type electrochemical immunosensor for cardiac troponin I based on β-cyclodextrin functionalized 3D graphene incorporated with Ag nanoclusters
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
Cardiac troponin I (cTnI) detection plays a critical role in the early diagnosis of acute myocardial infarction (AMI). In this study, a sandwich-type electrochemical immunosensor was developed for ultrasensitive quantification of cardiac troponin I (cTnI). The sensor platform utilized silver nanoclusters (AgNCs) incorporated with -cyclodextrin functionalized 3D porous graphene (-CD@3DG-AgNCs) to enhance electrical conductivity and primary antibody (Ab) immobilization. For signal amplification, ferrocenecarboxylic acid combined with -CD@3DG (-CD@3DG-Fc-COOH) was employed as the signal probe, which demonstrated excellent secondary antibody (Ab) capture capability. The synthesized nanomaterials were characterized using SEM, TEM, FTIR spectroscopy, X-ray Photoelectron Spectroscopy (XPS) and Thermo-Gravimetric Analysis (TGA). The electrochemical performance of the surface-modified electrodes was systematically investigated through cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Under optimized conditions, differential pulse voltammetry (DPV) revealed that the developed sensor exhibited an extensive linear response range (100 fg mL−1–100 ng mL−1) for cTnI quantification, with an exceptionally low detection limit of 45.5 fg mL−1. The recovery rate ranging from 97.39% to 102.83% was achieved in human serum analysis, suggesting the potential applicability of this immunosensor for clinical diagnosis.
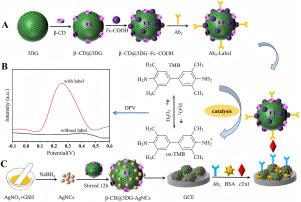
基于β-环糊精功能化三维石墨烯结合银纳米团簇的三明治型心肌肌钙蛋白I电化学免疫传感器
心肌肌钙蛋白I (cTnI)检测在急性心肌梗死(AMI)的早期诊断中起着至关重要的作用。本研究开发了一种三明治型电化学免疫传感器,用于超灵敏定量心肌肌钙蛋白I (cTnI)。该传感器平台利用银纳米团簇(agnc)结合β-环糊精功能化的三维多孔石墨烯(β-CD@3DG-AgNCs)来增强电导率和一抗(Ab1)固定。二茂铁羧酸结合β-CD@3DG (β-CD@3DG-Fc-COOH)作为信号探针,具有良好的二抗(Ab2)捕获能力。利用扫描电镜(SEM)、透射电镜(TEM)、红外光谱(FTIR)、x射线光电子能谱(XPS)和热重分析(TGA)对合成的纳米材料进行了表征。通过循环伏安法(CV)和电化学阻抗谱法(EIS)系统地研究了表面修饰电极的电化学性能。在优化条件下,差分脉冲伏安法(DPV)表明,所开发的传感器对cTnI定量具有广泛的线性响应范围(100 fg mL−1 - 100 ng mL−1),检测限极低,为45.5 fg mL−1。在人血清分析中,该免疫传感器的回收率为97.39% ~ 102.83%,提示该免疫传感器在临床诊断中的潜在适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


