Closed-loop electrical stimulation prevents focal epilepsy progression and long-term memory impairment
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
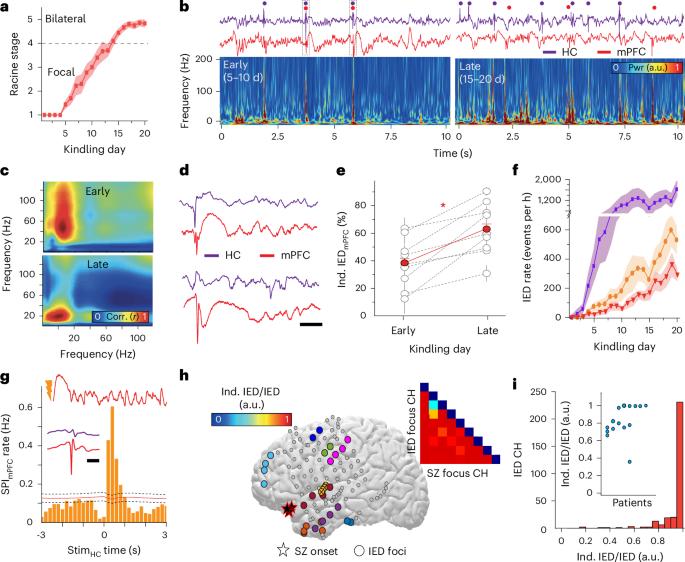
Interictal epileptiform discharges (IEDs) are expressed in epileptic networks and disrupt cognitive functions. It is unclear whether addressing IED-induced dysfunction could improve epilepsy outcomes, as most therapeutic approaches target seizures. We show, in a kindling model of progressive focal epilepsy, that IEDs produce pathological oscillatory coupling associated with prolonged, hypersynchronous neural spiking in synaptically connected cortex and expand the brain territory capable of generating IEDs. A similar relationship between IED-mediated oscillatory coupling and temporal organization of IEDs across brain regions was identified in human participants with refractory focal epilepsy. Spatiotemporally targeted closed-loop electrical stimulation triggered on hippocampal IED occurrence eliminated the abnormal cortical activity patterns, preventing the spread of the epileptic network and ameliorating long-term spatial memory deficits in rodents. These findings suggest that stimulation-based network interventions that normalize interictal dynamics may be an effective treatment of epilepsy and its comorbidities, with a low barrier to clinical translation. Pathological neural communication drives the spread of the epileptic network and contributes to memory impairment in focal epilepsy. The authors show that closed-loop electrical stimulation in rodents can prevent this interaction and preserve long-term memory.

闭环电刺激可预防局灶性癫痫进展和长期记忆障碍
癫痫样间期放电(ied)在癫痫网络中表达并破坏认知功能。目前尚不清楚解决ied诱导的功能障碍是否可以改善癫痫的结果,因为大多数治疗方法都是针对癫痫发作的。我们在进行性局灶性癫痫的点火模型中表明,在突触连接的皮层中,ied产生与长时间、超同步神经峰值相关的病理性振荡耦合,并扩大了能够产生ied的大脑区域。在难治性局灶性癫痫患者中,ied介导的振荡耦合和ied跨脑区域的时间组织之间也存在类似的关系。在海马IED发生时触发的时空定向闭环电刺激消除了异常的皮层活动模式,阻止了癫痫网络的扩散,改善了啮齿动物的长期空间记忆缺陷。这些发现表明,基于刺激的网络干预,使间歇期动态正常化,可能是癫痫及其合并症的有效治疗方法,临床转化障碍低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


