P-type ATPase magnesium transporter MgtA acts as a dimer
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Magnesium (Mg2+) uptake systems are present in all domains of life, consistent with the vital role of this ion. P-type ATPase Mg2+ importers are required for bacterial growth when Mg2+ is limiting or during pathogenesis. However, insights into their mechanisms of action are missing. Here we solved the cryo-EM structure of the Mg2+ transporter MgtA from Escherichia coli. We obtained high-resolution structures of both homodimeric (2.9 Å) and monomeric (3.6 Å) forms. The dimer structure is formed by multiple contacts between residues in adjacent soluble N and P subdomains. Our structures revealed an ion, assigned as Mg2+, in the transmembrane segment. Moreover, we detected two cytoplasmic ion-binding sites and determined the structure of the N-terminal tail. Sequence conservation, mutagenesis and ATPase assays indicate dimerization, the ion-binding sites and the N-terminal tail facilitate cation transport or serve regulatory roles. Zeinert et al. provide cryo-EM structures of the E. coli Mg2+ importer MgtA: unexpectedly, this P-type ATPase is a dimer with an uncommon transmembrane ion-binding site and knotted N-terminus, which are functionally important features.

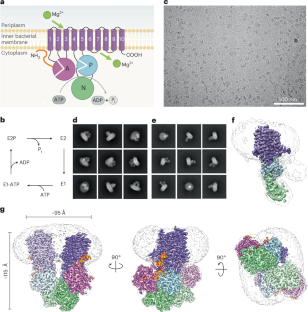
p型atp酶镁转运体MgtA作为二聚体
镁(Mg2+)摄取系统存在于生命的所有领域,与该离子的重要作用一致。当Mg2+限制或发病时,细菌生长需要p型atp酶Mg2+入口。然而,对其作用机制的深入了解尚不清楚。本文研究了大肠杆菌中Mg2+转运体MgtA的低温电镜结构。我们获得了同二聚体(2.9 Å)和单体(3.6 Å)形式的高分辨率结构。二聚体结构是由相邻可溶N和P子域残基之间的多次接触形成的。我们的结构揭示了一个离子,分配为Mg2+,在跨膜段。此外,我们检测到两个细胞质离子结合位点,并确定了n端尾部的结构。序列保存、突变和atp酶分析表明二聚体、离子结合位点和n端尾部促进阳离子运输或起调节作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


