Measurements of H2O-N2 line shape parameters and the determination of the intermolecular potential for complex Robert-Bonamy-Ma calculations
IF 1.9
3区 物理与天体物理
Q2 OPTICS
Journal of Quantitative Spectroscopy & Radiative Transfer
Pub Date : 2025-06-22
DOI:10.1016/j.jqsrt.2025.109570
引用次数: 0
Abstract
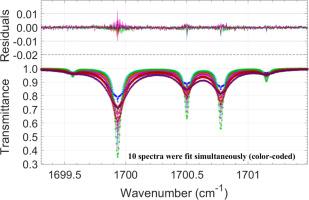
Ten sets of N2-mixture spectra of H2O were measured for the ν2 and 2ν2-ν2 bands in the 1200–1950 cm-1 region at room temperature using a straight-pass glass gas cell with KCl windows housed in the Bruker 125HR high-resolution Fourier transform spectrometer (FTS) at the Jet Propulsion Laboratory (JPL). The spectra were fit simultaneously using a multispectrum fitting software, which adopts a speed-dependent Voigt line shape profile having full line mixing effects taken into account through a relaxation matrix operation. N2-broadened half-width and N2-induced frequency shift coefficients were determined for 395 transitions of H216O. These data were then checked using the smooth variation and paring rules [Brown et al. J Mol Spectrosc. 2007;246:1–21, Ma et al. Mol Phys. 2011;109:1925–41] and 166 transitions with less than 3 % difference were chosen. Additional filtering was done using the air-broadened data of Birk and Wagner [JQSRT 2012;113, 889–928] and the H2O![]() O2 CRBM calculations of Gamache et al. [Mol. Phys. 2024;122: e2281592] to form air-broadening values for the measurements made here. The air-broadened values that agree with the Birk and Wagner data better than 3.0 % were retained. The list was augmented with 57 measurements that have γ less than 0.05 cm-1 and the pairing rules applied again. The final list contains 150 transitions. Using these data, Complex Robert-Bonamy-Ma calculations were made to determine the intermolecular potential for the H2O
O2 CRBM calculations of Gamache et al. [Mol. Phys. 2024;122: e2281592] to form air-broadening values for the measurements made here. The air-broadened values that agree with the Birk and Wagner data better than 3.0 % were retained. The list was augmented with 57 measurements that have γ less than 0.05 cm-1 and the pairing rules applied again. The final list contains 150 transitions. Using these data, Complex Robert-Bonamy-Ma calculations were made to determine the intermolecular potential for the H2O![]() N2 collision system. Starting from the potential of Vispoel et al. [JQSRT 2019;228:79]. potential parameters were changed iteratively until a final potential (potential RG23) was determined. The final results agree with the selected half-width measurements having an average difference of -1.67 %, an average absolute difference of 3.87 % and a standard deviation of 5.45 %. Calculations were then made for all ν2 transitions in the measurement database [Gamache and Hartmann, Can J Chem 2004;82:1013] to check the final intermolecular potential.
N2 collision system. Starting from the potential of Vispoel et al. [JQSRT 2019;228:79]. potential parameters were changed iteratively until a final potential (potential RG23) was determined. The final results agree with the selected half-width measurements having an average difference of -1.67 %, an average absolute difference of 3.87 % and a standard deviation of 5.45 %. Calculations were then made for all ν2 transitions in the measurement database [Gamache and Hartmann, Can J Chem 2004;82:1013] to check the final intermolecular potential.
H2O-N2线形参数的测量和配合物分子间电位的测定robert - bonami - ma计算
利用喷气推进实验室(JPL)的布鲁克125HR高分辨率傅立叶变换光谱仪(FTS)内装有KCl窗口的直通玻璃气池,在室温下测量了1200 ~ 1950 cm-1区域内ν2和2ν2-ν2波段的10组n2 - H2O混合光谱。多光谱拟合软件采用速度相关的Voigt线形轮廓,通过松弛矩阵运算考虑了全线混合效应。测定了H216O 395次跃迁的n2加宽半宽系数和n2诱导频移系数。然后使用平滑变化和配对规则检查这些数据[Brown等人]。中国生物医学工程学报,2007;26(1):1-2。Mol Phys. 2011;109:1925-41]和166个差异小于3%的跃迁。使用Birk和Wagner的空气展宽数据[JQSRT 2012;113, 889-928]和Gamache等人的H2O-O2 CRBM计算[Mol. Phys. 2024;122: e2281592]进行额外滤波,形成本文测量的空气展宽值。空气展宽值与Birk和Wagner数据的一致性优于3.0%。和配对规则再次应用。最终列表包含150个转换。利用这些数据,进行了复杂的robert - bonami - ma计算,以确定H2O-N2碰撞系统的分子间势。从Vispoel等人的潜力开始[JQSRT 2019;228:79]。反复改变电位参数,直至确定最终电位(电位RG23)。最终结果与所选半宽测量值一致,平均差值为%,平均绝对差值为%,标准差为5.45%。然后计算测量数据库中的所有ν2跃迁[Gamache and Hartmann, Can J Chem 2004;82:1013]以检查最终的分子间电位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.30
自引率
21.70%
发文量
273
审稿时长
58 days
期刊介绍:
Papers with the following subject areas are suitable for publication in the Journal of Quantitative Spectroscopy and Radiative Transfer:
- Theoretical and experimental aspects of the spectra of atoms, molecules, ions, and plasmas.
- Spectral lineshape studies including models and computational algorithms.
- Atmospheric spectroscopy.
- Theoretical and experimental aspects of light scattering.
- Application of light scattering in particle characterization and remote sensing.
- Application of light scattering in biological sciences and medicine.
- Radiative transfer in absorbing, emitting, and scattering media.
- Radiative transfer in stochastic media.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


