Within-host resistance evolution of ST15 Klebsiella pneumoniae in an ICU immunosuppressed patient under antibiotic pressure of polymyxins, ceftazidime-avibactam, and meropenem
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-06-19
DOI:10.1016/j.ijantimicag.2025.107554
引用次数: 0
Abstract
Objectives
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is a major pathogen in healthcare-associated infections, posing a severe and potentially fatal threat to critically ill patients. This study aims to investigate the evolution of antimicrobial resistance under the selective pressure from multiple antibiotics in immunocompromised patients in intensive care unit (ICU).
Methods
In this study, we report the complex dynamic evolution of resistance in ST15 CRKP in an immunocompromised critically ill patient, driven by adjustments in antibiotic regimens involving polymyxin, ceftazidime-avibactam (CZA), and meropenem. Antimicrobial susceptibility testing, whole-genome sequencing, and mutation analysis were performed on longitudinal clinical isolates.
Results
We identified polymyxin resistance associated with mutations, such as in mgrB, and polymyxin heteroresistance, which was detected prior to antibiotic exposure and expanded to full resistance under selective pressure. Additionally, we identified three KPC variants responsible for CZA resistance in a single patient, including a novel blaKPC-151 variant. The novel KPC-151 enzyme features a deletion of tyrosine (Y) at position 241 to threonine (T) at position 243, with a substitution of serine (S). Cloning experiments and enzyme kinetic measurements confirmed that KPC-151 confers resistance to ceftazidime-avibactam while restoring susceptibility to carbapenems. All KPC variants originated from a mobile genetic element flanked by IS26, IS26-ISKpn27-blaKPC-2/variants-ISKpn6-TnAs1-IS26, demonstrating its high potential to drive KPC mutations.
Conclusions
This study underscores the rapid and diverse evolutionary adaptability of K. pneumoniae under multiple antibiotic pressures in immunocompromised critically ill patients, emphasizing the need for dynamic monitoring of antimicrobial susceptibility testing and resistance gene mutations to guide antibiotic adjustments.
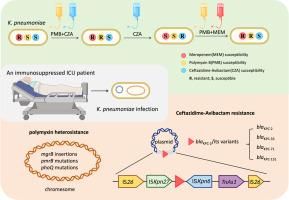
在多粘菌素、头孢他啶-阿维巴坦和美罗培南抗生素压力下,ICU免疫抑制患者ST15肺炎克雷伯菌的宿主内耐药性演变
耐碳青霉烯肺炎克雷伯菌(CRKP)是卫生保健相关感染的主要病原体,对危重患者构成严重和潜在致命的威胁。重症监护病房(icu)的免疫功能低下患者受到多种抗生素的选择性压力,这可能导致进一步的耐药性演变。在这项研究中,我们报告了在多粘菌素、头孢他啶-阿维巴坦(CZA)和美罗培南等抗生素治疗方案的调整下,一名免疫功能低下的危重患者ST15 CRKP耐药性的复杂动态演变。通过药敏试验、全基因组测序和纵向临床分离株的突变分析,我们确定了多粘菌素耐药性与突变相关,如mgrB和多粘菌素异源耐药,多粘菌素耐药在抗生素暴露前检测到,并在选择压力下扩展到完全耐药。此外,我们在单个患者中发现了三种导致CZA耐药的KPC变体,包括一种新的blaKPC-151变体。新的KPC-151酶的特点是在241位的酪氨酸(Y)缺失到243位的苏氨酸(T),并取代了丝氨酸(S)。克隆实验和酶动力学测定证实,KPC-151对头孢他啶-阿维巴坦具有抗性,同时恢复了对碳青霉烯类的敏感性。所有KPC变体都起源于IS26两侧的移动遗传元件IS26- iskpn27 - blakpc -2/ varients - iskpn6 - tnas1 -IS26,表明其具有驱动KPC突变的高潜力。本研究强调了免疫功能低下危重患者肺炎克雷伯菌在多种抗生素压力下的快速和多样化的进化适应性,强调了对抗生素药敏试验和耐药基因突变进行动态监测以指导抗生素调整的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


