Organosilicon molecules bind to the intrinsically disordered protein NUPR1 by clamping its hot-spots
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The nuclear protein 1, or NUPR1, is an intrinsically disordered protein (IDP) involved in the development and progression of pancreatic ductal adenocarcinoma (PDAC). We have previously developed drugs capable of binding at the two hot-spot regions of NUPR1, around residues Ala33 and Thr68, hampering its interactions in cellulo. In this work, we synthesized new organosilicon molecules targeting those key hot-spots. The compounds were obtained by an acid-catalyzed intramolecular cyclization of a starting alkenol that contains a silyl group attached to the double bond. Binding between the silyl compounds and NUPR1 involved the two hot-spots, as shown by 2D 1H–15N HSQC NMR. Molecular simulations clarified that the binding relies on a loose clamp mechanism of the ligands towards the hot-spots. The dissociation constants (Kd) were around 20 μM, as measured by several biophysical techniques. However, studies in cellulo with PDAC cells did not show a decrease of cell viability upon treatment with the compounds; furthermore, proximity ligation assays in cellulo with a natural partner protein of NUPR1, G3BP, did not show a significant level of interfering in such interaction when silyl compounds were present, probably due to the high hydrophobicity of the designed compounds. Thus, in the case of NUPR1, moderate-to-high drug binding affinities (Kd < 10 μM) in vitro and a higher hydrophilicity are necessary to hamper protein-protein interactions in cellulo. As a more general conclusion, in vitro binding of ligands to the protein hot-spots is a necessary condition in the drug design targeting IDPs, but it is not enough to guarantee inhibition of their interactions in cellulo.
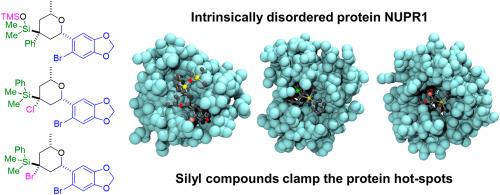
有机硅分子通过夹紧其热点与内在无序的蛋白质NUPR1结合。
核蛋白1 (NUPR1)是一种内在失调蛋白(IDP),参与胰腺导管腺癌(PDAC)的发生和进展。我们之前已经开发出能够结合NUPR1的两个热点区域的药物,在残基Ala33和Thr68周围,阻碍其在细胞中的相互作用。在这项工作中,我们针对这些关键热点合成了新的有机硅分子。这些化合物是由酸催化的起始烯醇分子内环化得到的,起始烯醇含有一个附着在双键上的硅基。硅基化合物与NUPR1之间的结合涉及两个热点,如2D 1H-15N HSQC NMR所示。分子模拟表明,这种结合依赖于配体对热点的松散夹紧机制。通过几种生物物理技术测量,解离常数(Kd)约为20 μM。然而,用PDAC细胞对纤维素进行的研究并未显示用化合物处理后细胞活力降低;此外,与NUPR1的天然伴侣蛋白G3BP在纤维素中的近距离连接实验显示,当存在硅基化合物时,这种相互作用没有受到显著的干扰,这可能是由于所设计的化合物的高疏水性。因此,在NUPR1的情况下,在体外中等至高的药物结合亲和度(Kd < 10 μM)和较高的亲水性是阻碍纤维素中蛋白质-蛋白质相互作用所必需的。作为一个更普遍的结论,配体与蛋白质热点的体外结合是针对IDPs的药物设计的必要条件,但不足以保证它们在细胞内的相互作用被抑制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


