Economic and effective regeneration of LiBH4 from its direct hydrolytic products using Al-rich alloys
IF 8.9
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
The hydrolysis of lithium borohydride (LiBH4) is very promising for on-site hydrogen generation to supply hydrogen since it has appealing features such as a high hydrogen capacity (13.9 wt%, water included), fast hydrogen evolution rate, and mild reaction conditions. The large-scale application of LiBH4, however, is restricted by its difficult regeneration from stable hydrolytic products (LiBO2·xH2O), which requires expensive reducing agents such as MgH2, high energy-consuming dehydration process for LiBO2·2H2O, and additional hydrogen input. Herein, we develop a facile method to directly regenerate LiBH4 from LiBO2·2H2O by ball milling with low-cost Al-rich alloys as reductants and Li2O as additives, balancing efficiency and economy well. Introducing Mg (or Ca) into Al to form Al-rich alloys optimizes the mechanical brittleness and chemical reducibility, which facilitate the crushing, collision, and reaction of reactants and the conversion of H+ to H−. The specific mechanism study illustrates that Mg in Al3Mg2 preferentially reacts and the in-situ derived Al with fresh surface and fine size is highly active for reductive regeneration of LiBH4. Besides, a little addition of Li2O suppresses the formation of “boron sink”, [B12H12]−, further increasing the yield of LiBH4. This work evidences the regeneration potential of Al-based reducing agents on metal borohydrides.
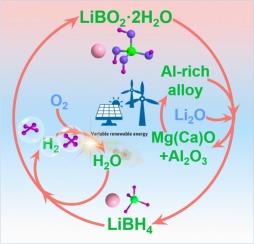
利用富铝合金经济有效地再生直接水解产物LiBH4
硼氢化锂(LiBH4)的水解具有高氢气容量(13.9 wt%,含水)、快速的氢气析出率和温和的反应条件等吸引人的特点,因此在现场制氢供应中非常有前景。然而,LiBH4的大规模应用受到其难以从稳定的水解产物(LiBO2·xH2O)中再生的限制,这需要昂贵的还原剂如MgH2, LiBO2·2H2O的高能耗脱水过程以及额外的氢输入。本研究以低成本富铝合金为还原剂,Li2O为添加剂,通过球磨直接从LiBO2·2H2O中再生LiBH4,实现了效率和经济性的平衡。在Al中加入Mg(或Ca)形成富Al合金,优化了合金的机械脆性和化学还原性,有利于破碎、碰撞和反应物的反应以及H+向H−的转化。具体机理研究表明,Mg在Al3Mg2中优先发生反应,原位衍生的Al表面新鲜、尺寸细小,对LiBH4的还原再生具有较高的活性。此外,少量Li2O的加入抑制了“硼汇”[B12H12]−的形成,进一步提高了LiBH4的产率。这项工作证明了铝基还原剂在金属硼氢化物上的再生潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of energy storage
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
11.80
自引率
24.50%
发文量
2262
审稿时长
69 days
期刊介绍:
Journal of energy storage focusses on all aspects of energy storage, in particular systems integration, electric grid integration, modelling and analysis, novel energy storage technologies, sizing and management strategies, business models for operation of storage systems and energy storage developments worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


