Eco-friendly electrochemical polishing of stainless steel using a NaCl-based electrolyte to reduce deterioration in seawater
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
One of the most significant technological challenges in implementing ocean energy conversion systems is ensuring the reliability and extending the service life of their components. Stainless steel has found widespread use in marine applications; however, it is susceptible to localized corrosion, erosion, and biocorrosion when exposed to seawater. In response to these challenges, selective electrodissolution has emerged as a promising strategy. Typically, the standard electrodissolution procedure is performed with acid-based electrolytes, generating environmental and safety problems. In this research, green electrolytes composed of ethylene glycol, sodium chloride, and water were used to selectively modify the surface of AISI 304 SS. Electrochemical behavior, wettability properties, surface characteristics, and corrosion resistance in simulated seawater were evaluated using cyclic voltammetry, contact angle, scanning electron microscopy and cyclic potentiodynamic polarization techniques, respectively. The anodic dissolution tests and their corresponding analysis indicated that the best surface modification was achieved by a potential of 4.0 V, a treatment time of 30 min, and an electrolyte of 10 wt. % NaCl, 67 wt. % EG and 23 wt. % H2O. Under these conditions, a hydrophobic surface was obtained, with a 72.2 % increase in resistance to pitting corrosion and a 71.8 % reduction in the general corrosion rate compared to untreated AISI 304. The incorporation of H2O into the EG solvent enhanced the selectivity of its electrodissolution process, reducing energy consumption and operational costs while increasing the environmental friendliness of the electrolyte.
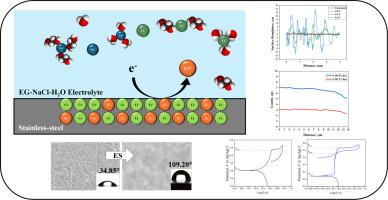

采用nacl基电解液对不锈钢进行环保电化学抛光,减少海水中的劣化
在实施海洋能源转换系统时,最重要的技术挑战之一是确保其组件的可靠性和延长使用寿命。不锈钢在船舶应用中得到了广泛的应用;然而,当暴露在海水中时,它容易受到局部腐蚀、侵蚀和生物腐蚀。为了应对这些挑战,选择性电溶解已成为一种有前途的策略。通常,标准的电溶解程序是用酸基电解质进行的,这会产生环境和安全问题。采用由乙二醇、氯化钠和水组成的绿色电解质对AISI 304 SS表面进行选择性修饰,分别采用循环伏安法、接触角法、扫描电镜和循环动电位极化技术对其在模拟海水中的电化学行为、润湿性、表面特性和耐腐蚀性进行了评价。阳极溶解试验及其分析表明,当电位为4.0 V,处理时间为30 min,电解液为10wt . % NaCl, 67wt . % EG和23wt . % H2O时,表面改性效果最佳。在这些条件下,获得了疏水表面,与未经处理的AISI 304相比,抗点蚀性提高了72.2%,总体腐蚀速率降低了71.8%。在EG溶剂中掺入H2O提高了其电溶解过程的选择性,降低了能耗和运行成本,同时提高了电解质的环境友好性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


