Cardiac intermediary metabolism in heart failure: substrate use, signalling roles and therapeutic targets
IF 44.2
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
The number of patients with heart failure is expected to rise sharply owing to ageing populations, poor dietary habits, unhealthy lifestyles and improved survival rates from conditions such as hypertension and myocardial infarction. Heart failure is classified into two main types: heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). These forms fundamentally differ, especially in how metabolism is regulated, but they also have shared features such as mitochondrial dysfunction. HFrEF is typically driven by neuroendocrine activation and mechanical strain, which demands a higher ATP production to sustain cardiac contraction. However, the primary energy source in a healthy heart (fatty acid β-oxidation) is often suppressed in HFrEF. Although glucose uptake increases in HFrEF, mitochondrial dysfunction disrupts glucose oxidation, and glycolysis and ketone oxidation only partially compensate for this imbalance. Conversely, HFpEF, particularly in individuals with metabolic diseases, such as obesity or type 2 diabetes mellitus, results from both mechanical and metabolic overload. Elevated glucose and lipid levels overwhelm normal metabolic pathways, leading to an accumulation of harmful metabolic byproducts that impair mitochondrial and cellular function. In this Review, we explore how disruptions in cardiac metabolism are not only markers of heart failure but also key drivers of disease progression. We also examine how metabolic intermediates influence signalling pathways that modify proteins and regulate gene expression in the heart. The growing recognition of the role of metabolic alterations in heart failure has led to groundbreaking treatments that target these metabolic disruptions, offering new hope for these patients. In this Review, Mericskay and colleagues discuss the role of cardiac intermediary metabolism in heart failure, describing the perturbations in cardiac energy metabolism that occur in heart failure with reduced ejection fraction and cardiometabolic heart failure with preserved ejection fraction, and highlighting potential treatments to target intermediary metabolism in these patients.
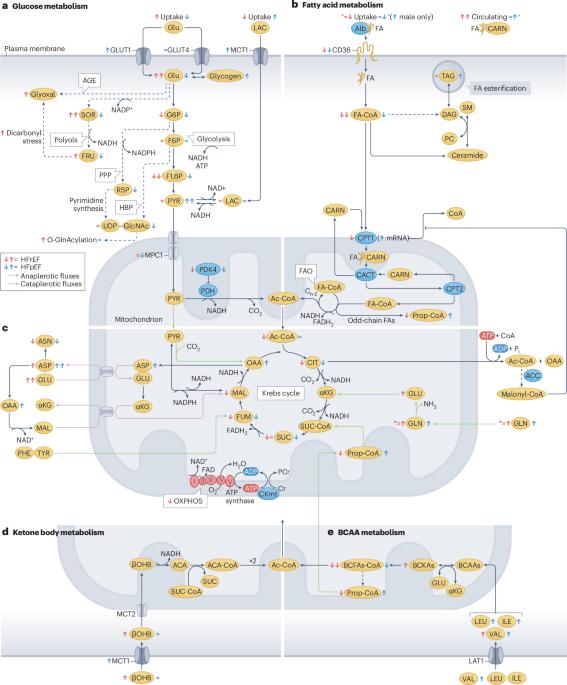
心力衰竭的心脏中间代谢:底物使用,信号作用和治疗靶点。
由于人口老龄化、不良的饮食习惯、不健康的生活方式以及高血压和心肌梗塞等疾病的存活率提高,心力衰竭患者的数量预计将急剧增加。心力衰竭主要分为两种类型:心力衰竭伴射血分数降低(HFrEF)和心力衰竭伴射血分数保持(HFpEF)。这些形式在本质上是不同的,尤其是在新陈代谢的调节方式上,但它们也有共同的特征,比如线粒体功能障碍。HFrEF通常由神经内分泌激活和机械应变驱动,这需要更高的ATP生成来维持心脏收缩。然而,健康心脏的主要能量来源(脂肪酸β-氧化)在HFrEF中经常被抑制。虽然HFrEF中葡萄糖摄取增加,但线粒体功能障碍会破坏葡萄糖氧化,糖酵解和酮氧化只能部分补偿这种不平衡。相反,HFpEF,特别是在患有代谢性疾病(如肥胖或2型糖尿病)的个体中,是机械和代谢超负荷的结果。葡萄糖和脂质水平升高会破坏正常的代谢途径,导致有害代谢副产物的积累,损害线粒体和细胞功能。在这篇综述中,我们探讨了心脏代谢紊乱不仅是心力衰竭的标志,也是疾病进展的关键驱动因素。我们还研究了代谢中间体如何影响改变蛋白质和调节心脏基因表达的信号通路。越来越多的人认识到代谢改变在心力衰竭中的作用,这导致了针对这些代谢紊乱的突破性治疗,为这些患者带来了新的希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cardiology
医学-心血管系统
CiteScore
53.10
自引率
0.60%
发文量
143
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cardiology aims to be the go-to source for reviews and commentaries in the scientific and clinical communities it serves. Focused on providing authoritative and accessible articles enriched with clear figures and tables, the journal strives to offer unparalleled service to authors, referees, and readers, maximizing the usefulness and impact of each publication. It covers a broad range of content types, including Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives, catering to practising cardiologists and cardiovascular research scientists. Authored by renowned clinicians, academics, and researchers, the content targets readers in the biological and medical sciences, ensuring accessibility across various disciplines. In-depth Reviews offer up-to-date information, while Consensus Statements provide evidence-based recommendations. Perspectives and News & Views present topical discussions and opinions, and the Research Highlights section filters primary research from cardiovascular and general medical journals. As part of the Nature Reviews portfolio, Nature Reviews Cardiology maintains high standards and a wide reach.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


