Role of FGF19 in regulating mitochondrial dynamics and macrophage polarization through FGFR4/AMPKα-p38/MAPK Axis in bleomycin-induced pulmonary fibrosis
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
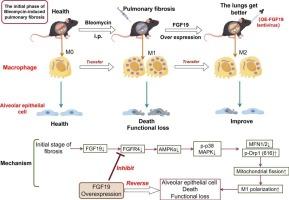
In the bleomycin (BLM)-induced pulmonary fibrosis model, macrophage polarization and mitochondrial dynamic imbalance are critical drivers of fibrogenesis. Although fibroblast growth factor 19 (FGF19) has been reported to alleviate fibrosis, its mechanism of regulating mitochondrial dynamics and macrophage polarization through the FGFR4/AMPKα-p38/MAPK axis remains unclear.
Objective
To investigate whether FGF19 mitigates alveolar epithelial injury and pulmonary fibrosis by restoring mitochondrial fusion/fission balance and modulating macrophage phenotype switching.
Methods
A BLM-induced C57BL/6 mouse fibrosis model was employed, with lung-specific FGF19 overexpression via lentivirus. An in vitro RAW264.7 macrophage-alveolar epithelial cell coculture system was used to assess mitochondrial morphology (transmission electron microscopy), mtDNA content (qPCR), protein expression (MFN1/2, Drp1-pSer616; Western blot), and macrophage polarization (flow cytometry). Pharmacological inhibition (SB203580, a p38/MAPK inhibitor) and MFN1/MFN2 siRNA knockdown were applied to validate pathway specificity.
Results
(1) FGF19 overexpression significantly attenuated BLM-induced alveolar destruction, collagen deposition, and inflammatory infiltration (H&E, P < 0.01); (2) FGF19 activated the FGFR4/AMPKα-p38/MAPK pathway, upregulated mitochondrial fusion proteins MFN1/2 (P < 0.01), suppressed Drp1 phosphorylation (Ser616)-mediated fission (P < 0.05), and shifted macrophages toward an M2 phenotype (CD206↑, P < 0.01); (3) p38/MAPK inhibition or MFN1/2 knockdown reversed FGF19-driven M2 polarization (P < 0.01); (4) FGF19 reduced alveolar epithelial apoptosis (Annexin V-FITC, P < 0.01) and inflammatory cytokine release (TNF-α, IL-6; ELISA, P < 0.01) by inhibiting M1 polarization.
Conclusion
FGF19 alleviates pulmonary fibrosis by restoring mitochondrial dynamics via the FGFR4/AMPKα-p38/MAPK axis, thereby inhibiting M1 macrophage polarization and epithelial injury. These findings highlight FGF19 as a potential therapeutic target for antifibrotic interventions.
FGF19通过FGFR4/AMPKα-p38/MAPK轴调控线粒体动力学和巨噬细胞极化在博莱霉素诱导的肺纤维化中的作用
在博来霉素(BLM)诱导的肺纤维化模型中,巨噬细胞极化和线粒体动态失衡是纤维化发生的关键驱动因素。尽管有报道称成纤维细胞生长因子19 (FGF19)可以缓解纤维化,但其通过FGFR4/AMPKα-p38/MAPK轴调节线粒体动力学和巨噬细胞极化的机制尚不清楚。目的探讨FGF19是否通过恢复线粒体融合/裂变平衡和调节巨噬细胞表型转换来减轻肺泡上皮损伤和肺纤维化。方法采用blm诱导C57BL/6小鼠纤维化模型,通过慢病毒介导肺特异性FGF19过表达。采用体外RAW264.7巨噬细胞-肺泡上皮细胞共培养系统评估线粒体形态(透射电镜)、mtDNA含量(qPCR)、蛋白表达(MFN1/2、Drp1-pSer616;Western blot)和巨噬细胞极化(流式细胞术)。药理抑制(SB203580,一种p38/MAPK抑制剂)和MFN1/MFN2 siRNA敲低验证通路特异性。结果(1)FGF19过表达显著减轻blm诱导的肺泡破坏、胶原沉积和炎症浸润(H&;E, P <;0.01);(2) FGF19激活FGFR4/AMPKα-p38/MAPK通路,上调线粒体融合蛋白MFN1/2 (P <;0.01),抑制Drp1磷酸化(Ser616)介导的裂变(P <;0.05),并将巨噬细胞向M2表型转移(CD206↑,P <;0.01);(3) p38/MAPK抑制或MFN1/2敲低可逆转fgf19驱动的M2极化(P <;0.01);(4) FGF19降低肺泡上皮细胞凋亡(Annexin V-FITC, P <;0.01)和炎性细胞因子释放(TNF-α、IL-6;ELISA, P <;0.01),抑制M1极化。结论fgf19通过FGFR4/AMPKα-p38/MAPK轴恢复线粒体动力学,从而抑制M1巨噬细胞极化和上皮损伤,减轻肺纤维化。这些发现强调了FGF19作为抗纤维化干预的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


