Metabolic network remodeling through PxJHE modulates temperature adaptation in a cosmopolitan insect
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Understanding the molecular mechanisms underlying temperature adaptation in agricultural pests is crucial for predicting their evolutionary responses to climate change. Here, we investigate the role of juvenile hormone esterase gene PxJHE in temperature adaptation of Plutella xylostella, a globally distributed pest. Spatial-temporal expression patterns demonstrates significantly reduced PxJHE transcript levels in hot-evolved (HS), and cold-evolved (CS) strains across all tested temperatures compared to ancestral strain (AS). CRISPR/Cas9-mediated knockout strains (JHE-MU) exhibit substantially elevated juvenile hormone (JH) titers during development, accompanied by impaired extreme temperature tolerance and altered life history parameters. Biochemical analyses reveal that PxJHE deficiency leads to significant accumulation of lipids and total sugars, while simultaneously reducing antioxidant enzyme activities (SOD and CAT). Metabolomic profiling reveal that PxJHE deficiency causes extensive metabolic rewiring, particularly in lipid, carbohydrate and amino acid pathways. Gene expression analysis demonstrates that PxJHE knockout downregulates key metabolic enzymes including 6-phosphofructokinase (PxPFK) and hormone-sensitive lipase (PxHSL), indicating impaired energy mobilization despite enhanced substrate storage. Our findings demonstrate that PxJHE modulates temperature adaptation through a multi-level regulatory mechanism involving JH signaling, metabolic network coordination, and antioxidant defense systems. This study provides novel insights into the genetic architecture of climate adaptation in agricultural pests, revealing the crucial interface between hormonal regulation, metabolic plasticity, and oxidative stress management in environmental adaptation.
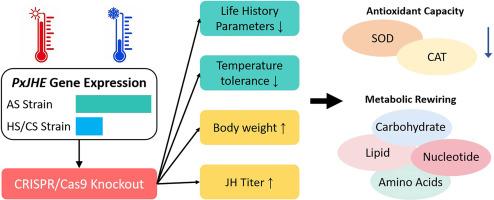
通过PxJHE代谢网络重塑调节世界性昆虫的温度适应
了解农业害虫温度适应的分子机制对于预测其对气候变化的进化反应至关重要。本文研究了幼体激素酯酶基因PxJHE在小菜蛾温度适应中的作用。时空表达模式显示,在所有测试温度下,与祖先菌株(AS)相比,热进化菌株(HS)和冷进化菌株(CS)的PxJHE转录水平显著降低。CRISPR/ cas9介导的敲除菌株(JHE-MU)在发育过程中表现出显著升高的幼体激素(JH)滴度,同时伴有极端温度耐受性受损和生活史参数改变。生化分析表明,PxJHE缺乏导致脂质和总糖的显著积累,同时降低抗氧化酶(SOD和CAT)活性。代谢组学分析显示PxJHE缺乏导致广泛的代谢重新布线,特别是在脂质,碳水化合物和氨基酸途径中。基因表达分析表明,PxJHE基因敲除下调了包括6-磷酸果糖激酶(PxPFK)和激素敏感脂肪酶(PxHSL)在内的关键代谢酶,表明尽管底物储存增强,但能量动员受损。我们的研究结果表明,PxJHE通过涉及JH信号、代谢网络协调和抗氧化防御系统的多层次调节机制来调节温度适应。该研究为农业害虫气候适应的遗传结构提供了新的见解,揭示了环境适应中激素调节、代谢可塑性和氧化应激管理之间的关键接口。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


