De novo biosynthesis of daptomycin through module construction and assembly in Escherichia coli
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
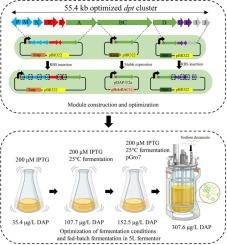
Daptomycin (DAP) is a non-ribosomal peptide (NRP) produced by Streptomyces roseosporus and the fermentation process is time-consuming and labor-intensive. Since non-ribosomal peptide synthetase assembles DAP from simple amino acid building blocks, the heterologous expression of DAP in a robust and easily manipulated host is an attractive strategy to enhance its accessibility. In this study, we rationally designed and refactored the 73.8-kb DAP biosynthesis gene cluster (dpt cluster). Subsequently, the optimized 55.4-kb dpt cluster was divided into three rationally designed modules to respectively clone into pET28a. The three modules were then co-transformed into E . coli BL21 (DE3) to assemble and achieve heterologous expression of DAP. We further optimized the highly repetitive codons of the dptBC gene and reduced the copy number of dptBC gene, thereby stabilizing the gene to ensure consistent production of DAP in E. coli. We also refactored the dpt cluster to improve the translation efficiency of the DAP synthetic protein. Finally, we optimized and determined the most suitable fermentation conditions for DAP production in E. coli, which resulted in DAP yield up to 307.60 μg/L in a 5 L bioreactor. Our study demonstrates the inaugural successful peptide module construction and assembly to achieve de novo heterologous biosynthesis of DAP in E. coli, which establishes a proof-of-concept technology system for the engineered production of structurally diverse NRPs in prokaryotic hosts, advancing synthetic biology frameworks for complex natural product assembly.
在大肠杆菌中通过模块构建和组装重新合成达托霉素
达托霉素(DAP)是一种由玫瑰孢链霉菌产生的非核糖体肽(NRP),其发酵过程耗时费力。由于非核糖体肽合成酶从简单的氨基酸构建块组装DAP,因此在健壮且易于操作的宿主中异源表达DAP是提高其可及性的一种有吸引力的策略。本研究合理设计并重构了73.8 kb的DAP生物合成基因簇(dpt簇)。随后,将优化后的55.4 kb dpt集群划分为三个合理设计的模块,分别克隆到pET28a中。然后将这三个模块共变换为E。大肠杆菌BL21 (DE3)组装并实现DAP的异源表达。我们进一步优化了dptBC基因的高重复密码子,减少了dptBC基因的拷贝数,从而稳定了该基因,保证了DAP在大肠杆菌中的一致性生产。我们还重构了dpt簇,以提高DAP合成蛋白的翻译效率。最后,我们优化并确定了在大肠杆菌中生产DAP的最适宜发酵条件,在5 L的生物反应器中,DAP的产率可达307.60 μg/L。我们的研究首次成功构建和组装肽模块,在大肠杆菌中实现了DAP的从头异源生物合成,这为在原核生物宿主中工程生产结构多样化的nrp建立了概念验证技术体系,推进了复杂天然产物组装的合成生物学框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


