A survey on manipulating association/dissociation rate constants of electrochemical aptamer-based biosensors for thrombin analysis
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The accurate analysis of protein plays a significant role in disease diagnosis and treatment. Electrochemical aptamer-based (EAB) sensors, known for their high sensitivity and cost-effectiveness, are good candidates for protein analysis. Despite of their advantages, there is a lack of the systematical study on optimized conditions for protein analysis. For instance, the association and dissociation rate constants (kon and koff), the binding kinetics (e.g., τ) and thermodynamics (KD value) are of significance for sensing purpose, which are greatly impacted by several parameters including probe density, temperatures, or salt concentrations. Here, we employ thrombin as a test bed to investigate the sensing performance dependence on these three parameters. We achieved an optimal probe density of 10 nm to support a robust binding event across the probe distance range of 8 nm–62 nm. For temperature condition, we observed that at 45 °C sensors exhibited highest values for both kon and koff constants, resulting a moderate binding affinity. The optimal temperature for the overall sensor performance remains as 37 °C. Finally, we demonstrated that the protein analysis is greatly dependent of salt concentrations as well as the valency, with the addition of 20 mM Mg2+ exhibiting a significant increase of both kon and koff values. The findings contribute to advancing biosensor technology and expanding its applications in biological research, holding promise for future developments in protein detection and analysis.
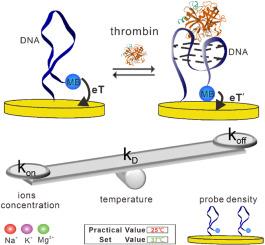
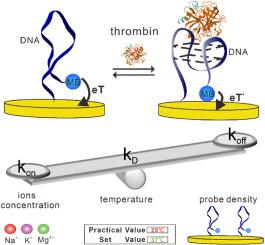
控制电化学配体生物传感器在凝血酶分析中的结合/解离速率常数的研究进展
蛋白质的准确分析对疾病的诊断和治疗具有重要意义。基于电化学适体(EAB)的传感器以其高灵敏度和高性价比而闻名,是蛋白质分析的良好候选者。尽管它们具有优势,但对蛋白质分析的优化条件缺乏系统的研究。例如,缔合和解离速率常数(kon和koff)、结合动力学(例如τ)和热力学(KD值)对传感目的具有重要意义,它们受到探针密度、温度或盐浓度等几个参数的极大影响。在这里,我们采用凝血酶作为测试平台来研究这三个参数对传感性能的依赖。我们实现了10 nm的最佳探针密度,以支持在8 nm-62 nm的探针距离范围内的强大结合事件。在温度条件下,我们观察到传感器在45°C时显示出最高的kon和koff常数,从而产生中等的结合亲和力。整体传感器性能的最佳温度保持在37°C。最后,我们证明了蛋白质分析很大程度上依赖于盐浓度和价态,添加20 mM Mg2+显示kon和koff值显着增加。这一发现有助于推进生物传感器技术并扩大其在生物学研究中的应用,为蛋白质检测和分析的未来发展带来希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


