A holistic science-based approach to container closure integrity for parenteral products: Lessons learned from a vaccine requiring deep cold storage using a conventional vial system
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-18
DOI:10.1016/j.ejpb.2025.114791
引用次数: 0
Abstract
The increasing implementation of deterministic analytical methods for container closure integrity (CCI) testing has enabled deeper insight into the closure performance of parenteral primary packaging. However, a strategy for ensuring CCI of sterile injectable product goes beyond CCI testing. A science-based holistic approach that includes identification of the risk to CCI, robust design of the primary packaging components & qualification of the container sealing/assembly process, and the implementation of appropriate process controls is required. This paper describes the implementation of such an approach for a vaccine stored at deep cold storage and lessons that can be applied to parenteral vial product in general to ensure that both the primary packaging design and the process contribute to assurance of CCI.
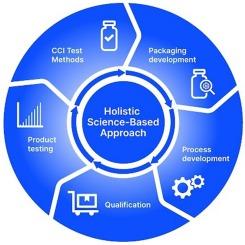
以科学为基础的对非注射产品容器封闭完整性的整体方法:从需要使用传统小瓶系统进行深度冷藏的疫苗中吸取的经验教训
越来越多的容器封闭完整性(CCI)测试的确定性分析方法的实施已经能够更深入地了解非注射初级包装的封闭性能。然而,确保无菌注射产品CCI的策略不仅仅是CCI测试。以科学为基础的整体方法,包括识别CCI风险,主要包装组件的稳健设计;对容器密封/装配过程进行确认,并实施适当的过程控制。本文描述了在深冷库中储存的疫苗的这种方法的实施情况,以及一般适用于注射小瓶产品的经验教训,以确保初级包装设计和工艺都有助于保证CCI。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


