Supercritical CO2 extraction behavior of electrolyte solvents from Li-ion battery black mass
IF 8.4
2区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
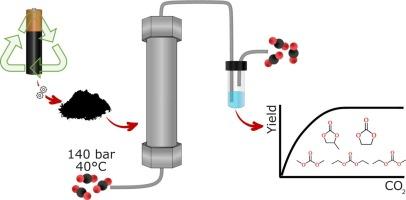
Electrolyte recovery from spent Li-ion batteries remains a significant challenge in the current recycling process. Li-ion battery waste streams containing electrolyte residues are classified as hazardous waste and entail a financial and workplace safety burden for the recycling industry. Recent studies show the potential use of supercritical CO2 extraction for the recovery of electrolyte solvents. In this study, the extraction behavior of electrolyte solvents from Li-ion battery black mass using supercritical CO2 process under pressures of 100 and 140 bar at 40°C was investigated. The extraction yield of dimethyl carbonate, ethyl methyl carbonate, and diethyl carbonate exceeded 99 % at both pressures. Ethylene carbonate, biphenyl, and propylene carbonate were successfully extracted with an extraction yield exceeding 95 % using 140 bar and 40°C. The extraction rates of biphenyl, ethylene carbonate and propylene carbonate at 140 bar and 40°C in the linear extraction regime of the extraction curve were determined to be 0.18 mg/g CO2, 1.9 mg/g CO2 and 0.4 mg/g CO2, respectively. The research demonstrates that supercritical CO₂ processing is a highly promising method not only for recycling electrolytes but also for mitigating the hazardous risks associated with battery waste.
锂离子电池黑质量电解液溶剂超临界CO2萃取行为研究
从废旧锂离子电池中回收电解液仍然是当前回收过程中的一个重大挑战。含有电解质残留物的锂离子电池废物流被归类为危险废物,给回收行业带来了财政和工作场所安全负担。近年来的研究表明,超临界CO2萃取在电解液溶剂的回收中具有潜在的应用前景。在40℃条件下,研究了超临界CO2工艺在100和140 bar压力下,从锂离子电池黑质量中提取电解质溶剂的行为。在两种压力下,碳酸二甲酯、碳酸甲酯和碳酸二乙酯的提取率均超过99% %。在140 bar和40°C条件下,成功地提取了碳酸乙烯、联苯和碳酸丙烯,提取率超过95 %。在140 bar和40°C条件下,联苯、碳酸乙烯和碳酸丙烯的提取率分别为0.18 mg/g CO2、1.9 mg/g CO2和0.4 mg/g CO2。研究结果表明,超临界CO₂处理不仅可以回收电解液,还可以减少电池废弃物带来的危险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of CO2 Utilization
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.90
自引率
10.40%
发文量
406
审稿时长
2.8 months
期刊介绍:
The Journal of CO2 Utilization offers a single, multi-disciplinary, scholarly platform for the exchange of novel research in the field of CO2 re-use for scientists and engineers in chemicals, fuels and materials.
The emphasis is on the dissemination of leading-edge research from basic science to the development of new processes, technologies and applications.
The Journal of CO2 Utilization publishes original peer-reviewed research papers, reviews, and short communications, including experimental and theoretical work, and analytical models and simulations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


