TNF switches homeostatic efferocytosis to lytic caspase-8–dependent pyroptosis and IL-1β maturation
IF 16.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
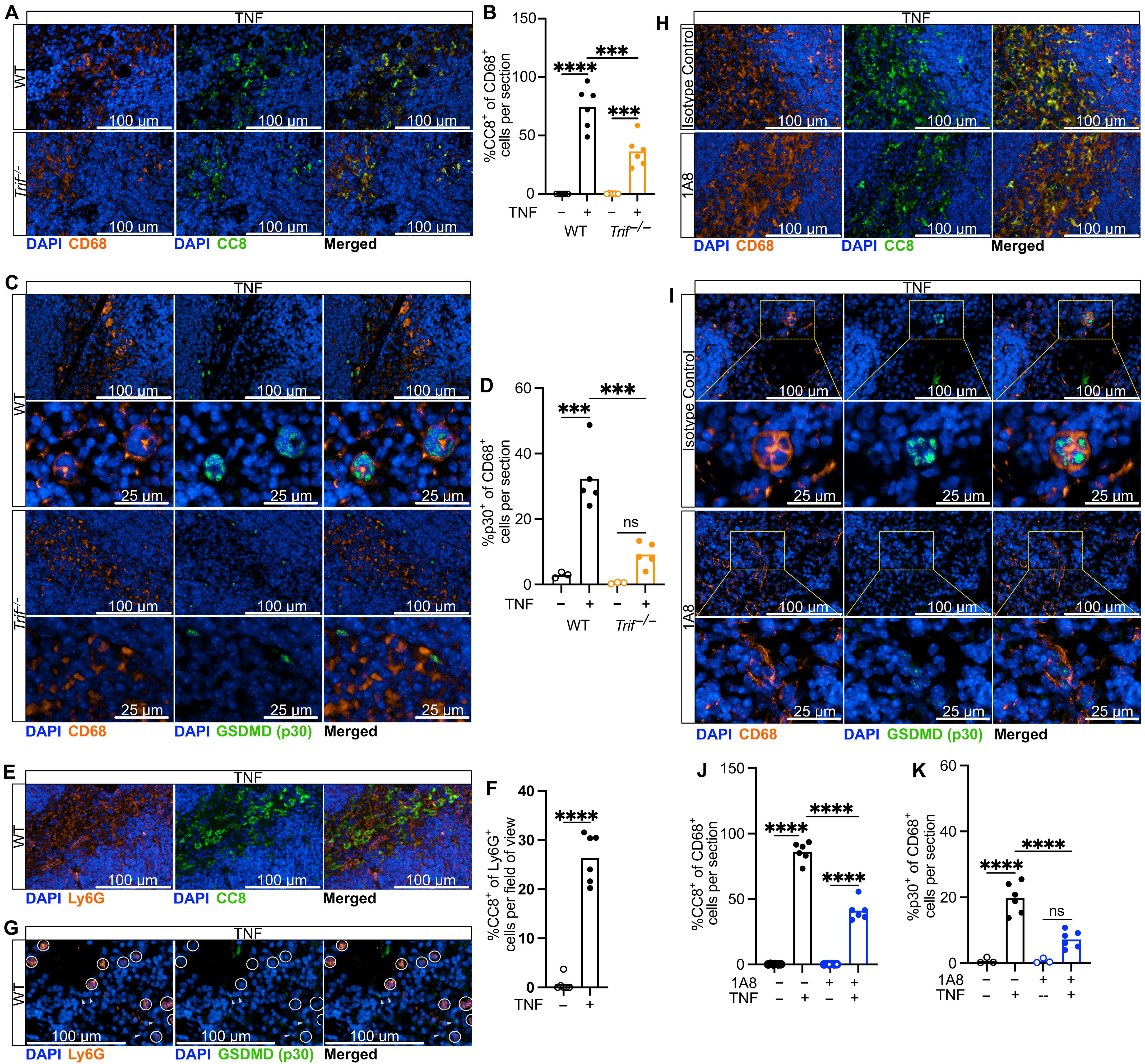
Efferocytosis, wherein phagocytes engulf dead or dying cells, is a critical function of macrophages that supports cellular turnover, tissue repair, and resolution of inflammation. Despite its well-established anti-inflammatory mechanism in homeostasis, whether efferocytosis remains immunologically silent in the context of dysregulated immune responses such as sepsis or systemic inflammatory response syndrome (SIRS) has not been investigated. Here, we used mouse models of tumor necrosis factor (TNF)–induced SIRS and Escherichia coli–induced septic peritonitis to uncover a potential negative consequence of efferocytosis. We found that when activated with TNF, phagocytes efferocytosing neutrophils initiated a caspase-8–dependent, but NLRP3 inflammasome–independent, form of pyroptosis, which we termed “efferoptosis.” The maturation of IL-1β, a hallmark of pyroptotic cell death, also occurred independently of canonical inflammasome activation, supporting direct cleavage by caspase-8. Inhibition of efferocytosis protected mice against TNF-induced SIRS, suggesting that efferoptosis contributes to the pathology of sepsis and other TNF-mediated inflammatory conditions.
TNF将稳态efferocytosis转换为裂解caspase-8依赖性焦亡和IL-1β成熟
巨噬细胞吞噬死亡或濒死细胞的Efferocytosis是巨噬细胞支持细胞更新、组织修复和炎症消退的关键功能。尽管在体内平衡中具有良好的抗炎机制,但在脓毒症或全身性炎症反应综合征(SIRS)等免疫反应失调的情况下,efferocytosis是否保持免疫沉默尚未得到研究。在这里,我们使用肿瘤坏死因子(TNF)诱导的SIRS和大肠杆菌诱导的脓毒性腹膜炎小鼠模型来揭示efferocytosis的潜在负面后果。我们发现,当被TNF激活时,吞噬细胞向嗜中性粒细胞产生一种依赖caspase-8,但与NLRP3炎症小体无关的焦亡形式,我们称之为“efferoptosis”。作为热亡细胞死亡的标志,IL-1β的成熟也独立于典型炎性体的激活而发生,支持caspase-8的直接裂解。抑制efferocysis可以保护小鼠免受tnf诱导的SIRS,这表明efferocysis有助于败血症和其他tnf介导的炎症条件的病理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


