Exploring the degradation mechanism of bisphenol-A with low Fe2+&H2O2 content and the toxicity evolution of oxidation intermediates
IF 8.7
Q1 Environmental Science
引用次数: 0
Abstract
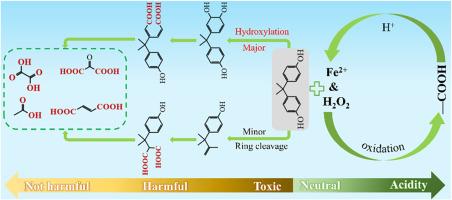
Efficient and cost-effective removal of emerging contaminants from water bodies has become a global priority. This study assessed the degradation efficiency of bisphenol-A (BPA) at varying Fe2+&H2O2 (molar ratio 1:1) concentrations. In addition, the degradation mechanism of BPA by Fe2+&H2O2 and the evolution of toxicity in intermediates were examined. The results indicated that, no additional acid is needed, with a Fe2+&H2O2 concentration at 0.15 mM and a reaction time of 20 min, and the degradation efficiency of BPA exceeded 90%; this is primarily because the oxidation still occurred when Fe2+&H2O2 was added to neutral BPA solutions, possibly leading to the conversion of the active functional groups of BPA into carboxyl groups. The deprotonation of these carboxyl groups provided the acidic conditions necessary for Fenton oxidation, thereby possibly facilitating further degradation of BPA and the formation of more carboxyl-rich intermediates, replenishing acidity for Fenton reactions and promoting continuous oxidation. Moreover, highly toxic intermediates are likely to form during the degradation of BPA at a 0.05–0.1 mM Fe2+&H2O2 concentrations, but this tendency decreases substantially with increasing Fe2+&H2O2 concentrations. At a Fe2+&H2O2 concentration of 0.2 mM, the intermediates were predominantly low-toxicity or even nontoxic. These findings highlight the importance of monitoring toxicity evolution and the conditions favorable for the formation of low-toxic or nontoxic intermediates in the removal of emerging contaminants. To further validate the applicability of this method, p-chloroxylenol (PCMX) and paracetamol (PAM) were also tested under identical conditions, and both showed high degradation efficiencies under neutral pH.
探讨低Fe2+和h2o2含量双酚a的降解机理及氧化中间体的毒性演变
从水体中高效和经济地去除新出现的污染物已成为全球的优先事项。本研究评估了不同Fe2+&;H2O2(摩尔比1:1)浓度下双酚a (BPA)的降解效率。此外,还考察了Fe2+& H2O2对BPA的降解机理及中间体毒性的演变。结果表明,在Fe2+&;H2O2浓度为0.15 mM,反应时间为20 min的条件下,无需添加酸,BPA的降解效率可达90%以上;这主要是因为在中性BPA溶液中加入Fe2+& & H2O2后,仍会发生氧化反应,可能导致BPA的活性官能团转化为羧基。这些羧基的去质子化为Fenton氧化提供了必要的酸性条件,从而可能促进BPA的进一步降解,形成更多富含羧基的中间体,为Fenton反应补充酸度,促进持续氧化。在0.05 ~ 0.1 mM Fe2+&;H2O2浓度下,BPA降解过程中可能形成高毒性中间体,但随着Fe2+&;H2O2浓度的增加,这一趋势显著降低。在Fe2+& H2O2浓度为0.2 mM时,中间体主要是低毒甚至无毒的。这些发现强调了监测毒性演变的重要性,以及在去除新出现的污染物时有利于形成低毒或无毒中间体的条件。为了进一步验证该方法的适用性,在相同的条件下对氯二酚(PCMX)和对乙酰氨基酚(PAM)也进行了测试,两者在中性pH下均表现出较高的降解效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Cycle
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
20
审稿时长
45 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


