Efficacy and Safety of Allisartan Isoproxil/Amlodipine in Patients with Essential Hypertension: A Phase III, Multicenter, Double-Blind, Parallel-Group, Randomised study
IF 3.4
4区 医学
Q2 PERIPHERAL VASCULAR DISEASE
引用次数: 0
Abstract
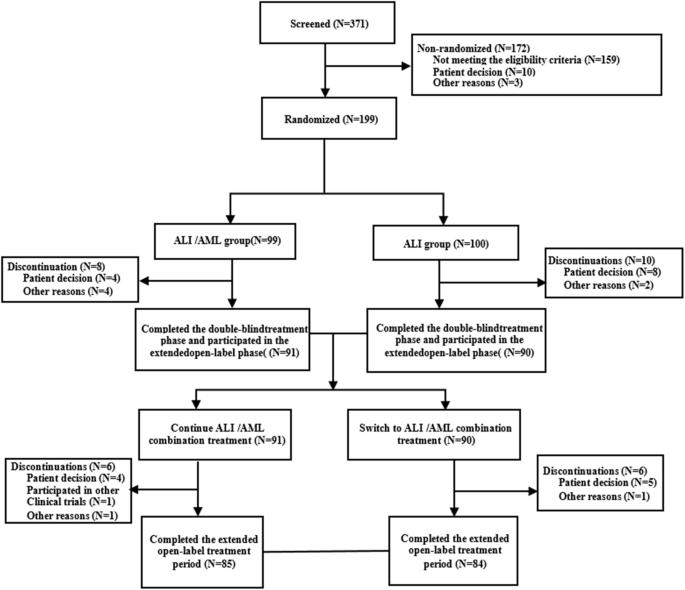
This multicenter, double-blind, parallel-group, randomised controlled phase III study evaluated the efficacy and safety of the Allisartan Isoproxil 240 mg /Amlodipine 5 mg (ALI/AML) combination compared with ALI 240 mg monotherapy in patients with mild-to-moderate essential hypertension. Patients aged 18 to 70 years with mean sitting systolic blood pressure (msSBP) between 140 and <180 mmHg and mean sitting diastolic blood pressure (msDBP) between 90 and <110 mmHg were randomised 1:1 to receive ALI/AML or ALI once-daily for 12 weeks after a 4-week treatment with ALI, followed by an open-label extension period with ALI/AML up to week 52. A total of 199 patients were randomised (ALI/AML: n = 99, ALI: n = 100) with 169 completing the study. Baseline characteristics were comparable between groups. After 12 weeks of randomisation, the reduction in msSBP (primary endpoint) was significantly greater in the ALI/AML group vs the ALI group (−18.3 vs. −9.3 mmHg, p < 0.001). Reductions in msDBP (−6.0 vs. −1.9 mmHg, p < 0.001) and 24-hour mean ambulatory systolic/diastolic blood pressure (−19.9/−10.1 vs. −6.9/−4.2 mmHg) were more pronounced in the ALI/AML group. Additionally, a greater proportion of patients achieved the BP response and target office BP in the ALI/AML group compared to the ALI group (53.6% vs. 25.5%, p < 0.001; 40.2% vs. 20.4%, p=0.0026). AML/ALI combination was generally safe and well tolerated, with sustained efficacy up to 52 weeks. The study concluded that ALI/AML offers a convenient, single-pill option for effective BP reduction in hypertensive patients.
阿利沙坦异丙醇/氨氯地平治疗原发性高血压的疗效和安全性:一项多中心、双盲、平行组、随机研究
这项多中心、双盲、平行组、随机对照的III期研究评估了阿利沙坦异丙醇240 mg /氨氯地平5 mg (ALI/AML)联合治疗与ALI 240 mg单药治疗在轻中度原发性高血压患者中的疗效和安全性。患者年龄18 ~ 70岁,平均坐位收缩压(msSBP)在140 ~ 140之间
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Human Hypertension
医学-外周血管病
CiteScore
5.20
自引率
3.70%
发文量
126
审稿时长
6-12 weeks
期刊介绍:
Journal of Human Hypertension is published monthly and is of interest to health care professionals who deal with hypertension (specialists, internists, primary care physicians) and public health workers. We believe that our patients benefit from robust scientific data that are based on well conducted clinical trials. We also believe that basic sciences are the foundations on which we build our knowledge of clinical conditions and their management. Towards this end, although we are primarily a clinical based journal, we also welcome suitable basic sciences studies that promote our understanding of human hypertension.
The journal aims to perform the dual role of increasing knowledge in the field of high blood pressure as well as improving the standard of care of patients. The editors will consider for publication all suitable papers dealing directly or indirectly with clinical aspects of hypertension, including but not limited to epidemiology, pathophysiology, therapeutics and basic sciences involving human subjects or tissues. We also consider papers from all specialties such as ophthalmology, cardiology, nephrology, obstetrics and stroke medicine that deal with the various aspects of hypertension and its complications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


