Pharmaceutical medicine advertising regulations in Australia, Japan, the United States, and India: An overview
Q3 Medicine
引用次数: 0
Abstract
Background
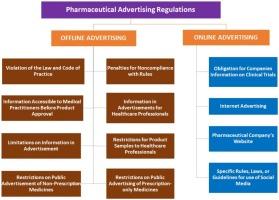
The advertising of pharmaceuticals is regulated by specific laws and codes of practice in Australia, Japan, the United States, and India.
Objective
The present review focuses on the similarities and differences in the regulations on advertisements for the promotion of pharmaceuticals in Australia, Japan, the United States, and India.
Methods
The pharmaceutical advertising regulations for four regions were collected, compiled, and compared to study the similarities and differences therein.
Results
If an advertisement is issued in breach of the code of practice and law, it is looked after by regulators. The company faces various penalties for non-compliance with the advertisement governing rules. The information required in an advertisement targeting healthcare providers and consumers is strictly regulated and restricted.
Conclusion
There is country to country variations in restrictions imposed over advertising non-prescription, and prescription medicines to the public. Moreover, regulatory agencies have implemented precise laws, rules, and guidance to control social media usage by pharmaceutical companies for advertising purposes.
澳大利亚、日本、美国和印度的医药广告法规:综述
在澳大利亚、日本、美国和印度,药品广告受到特定法律和行为准则的监管。目的综述澳大利亚、日本、美国和印度在药品促销广告法规方面的异同。方法收集、整理、比较4个地区药品广告法规的异同。结果如果广告的发布违反了业务守则和法律,它将受到监管机构的监管。该公司因不遵守广告管理规定而面临各种处罚。针对医疗保健提供者和消费者的广告所要求的信息受到严格监管和限制。结论各国对非处方药和处方药的广告限制存在差异。此外,监管机构已经实施了精确的法律、规则和指导,以控制制药公司出于广告目的使用社交媒体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Ethics, Medicine and Public Health
Medicine-Health Policy
CiteScore
2.20
自引率
0.00%
发文量
107
审稿时长
42 days
期刊介绍:
This review aims to compare approaches to medical ethics and bioethics in two forms, Anglo-Saxon (Ethics, Medicine and Public Health) and French (Ethique, Médecine et Politiques Publiques). Thus, in their native languages, the authors will present research on the legitimacy of the practice and appreciation of the consequences of acts towards patients as compared to the limits acceptable by the community, as illustrated by the democratic debate.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


