Examining green and chemical methods for zero-valent iron nanoparticle synthesis in heavy metal adsorption
IF 8.7
Q1 Environmental Science
引用次数: 0
Abstract
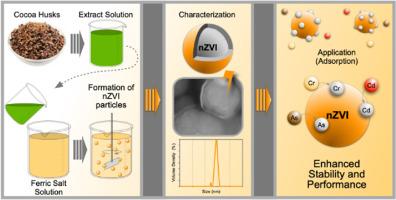
The increasing concern over heavy metal contamination in water has necessitated the development of sustainable and efficient treatment methods. This study compares two synthesis approaches for zero-valent iron nanoparticles (nZVI) for cadmium, chromium, and arsenic removal: chemical reduction using sodium borohydride and green synthesis utilizing cocoa husk extracts combined with hydrothermal carbonization (HTC). Chemically synthesized nZVI exhibited high initial removal efficiencies (>98%), though desorption effects occurred over time due to particle aging. In contrast, green-synthesized nZVI, stabilized by a carbon matrix, maintained consistent removal efficiencies above 98% for 120 h under acidic conditions, showcasing superior stability and reactivity. Characterization through SEM, EDS, and XRD confirmed the dual mechanisms of metal removal: reduction and adsorption facilitated by the Fe(0) core and oxide layers. While experimental conditions were optimized for each synthesis method, the findings highlight the promise of green-synthesized nZVI as a sustainable alternative for heavy metal remediation. Future studies should explore adsorption isotherms and long-term applications to further validate the scalability and efficacy of these materials.
重金属吸附合成零价铁纳米颗粒的绿色化学方法研究
随着人们对水中重金属污染的日益关注,有必要开发可持续和高效的处理方法。本研究比较了零价铁纳米颗粒(nZVI)去除镉、铬和砷的两种合成方法:使用硼氢化钠化学还原和使用可可壳提取物结合水热碳化(HTC)的绿色合成。化学合成的nZVI表现出很高的初始去除效率(98%),尽管随着时间的推移,由于颗粒老化,解吸效应会发生。相比之下,绿色合成的nZVI通过碳基质稳定,在酸性条件下保持120小时98%以上的稳定去除效率,表现出优异的稳定性和反应性。通过SEM、EDS和XRD表征,证实了Fe(0)芯层和氧化层促进金属的还原和吸附的双重去除机制。虽然每种合成方法的实验条件都得到了优化,但研究结果强调了绿色合成nZVI作为重金属修复的可持续替代方案的前景。未来的研究应探索吸附等温线和长期应用,以进一步验证这些材料的可扩展性和有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Cycle
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
20
审稿时长
45 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


