Feedback between F-actin organization and active stress governs criticality and energy localization in the cell cytoskeleton
IF 18.4
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
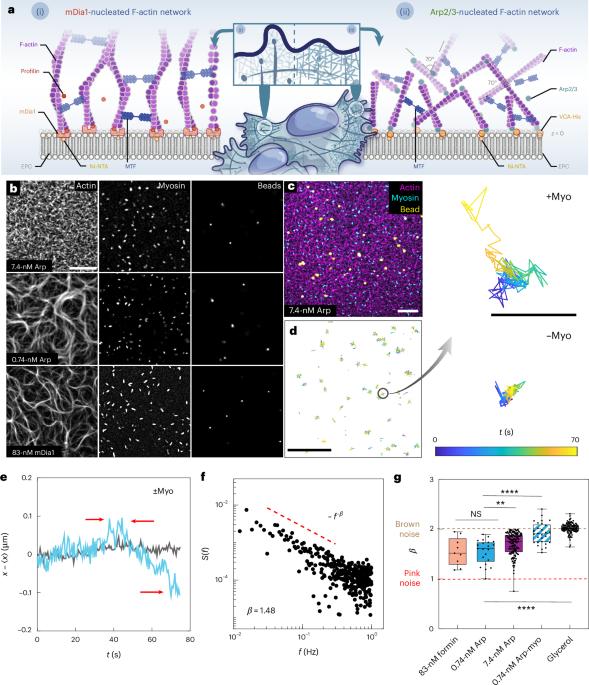
Self-organized criticality can occur in earthquakes, avalanches and biological processes, and is characterized by intermittent, scale-free energy dissipation. In living cells, the actin cytoskeleton undergoes dynamic structural reorganization, particularly during migration and division, where molecular motors generate mechanical stresses that drive large dissipative events. However, the mechanisms governing these critical transitions remain unclear. Here we show that cytoskeletal criticality emerges from the interplay between F-actin organization and active stress generation. Our study focuses on a minimal actomyosin system in vitro, which is composed of F-actin filaments, myosin II motors and nucleation-promoting factors. By systematically varying the actin connectivity and nematic order, we demonstrate that ordered and sparsely connected networks exhibit exponential stress dissipation, whereas disordered and highly connected networks show heavy-tailed distributions of energy release and the 1/f noise characteristic of self-organized criticality. Increased disorder leads to stress localization, shifting force propagation into stiffer mechanical modes, reminiscent of Anderson localization in condensed-matter systems. Furthermore, we show that network architecture directly regulates the myosin II filament size, establishing a chemical–mechanical feedback loop that modulates criticality. Our findings provide insights into the collective cytoskeletal dynamics, energy localization and cellular self-organization. Self-organized criticality can occur in cellular systems, but its origins remain unclear. Now it is shown that cytoskeletal criticality is influenced by the F-actin architecture and myosin active stress.

f -肌动蛋白组织和主动应激之间的反馈决定了细胞骨架的临界性和能量定位
自组织临界可以发生在地震、雪崩和生物过程中,其特征是间歇性的、无标度的能量耗散。在活细胞中,肌动蛋白细胞骨架经历动态结构重组,特别是在迁移和分裂过程中,分子马达产生机械应力,驱动大耗散事件。然而,控制这些关键转变的机制仍不清楚。在这里,我们表明细胞骨架的临界出现在f -肌动蛋白组织和主动应激产生之间的相互作用。我们的研究重点是在体外建立一个由F-actin丝、myosin II马达和成核促进因子组成的最小肌动球蛋白系统。通过系统地改变肌动蛋白连通性和向列顺序,我们证明了有序和稀疏连接的网络表现出指数级的应力耗散,而无序和高度连接的网络表现出能量释放的重尾分布和自组织临界的1/f噪声特征。增加的无序导致应力局部化,将力传播到更硬的机械模式,使人想起凝聚态系统中的安德森局部化。此外,我们表明网络结构直接调节肌凝蛋白II丝的大小,建立一个化学-机械反馈回路,调节临界。我们的发现提供了对集体细胞骨架动力学,能量定位和细胞自组织的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


