Transformation of Atrazine on δ-MnO2 Surfaces under Dry Conditions: Hydrolysis and Dealkylation
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
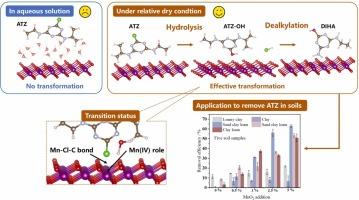
Atrazine (ATZ) is a frequently detected herbicide in corn fields, and manganese oxides (MnO2) are reactive soil components. Reportedly, the sole use of MnO2 cannot efficiently achieve ATZ removal in aqueous solution. However, this study found that 90.2% of ATZ could be degraded on δ-MnO2 surfaces under dry conditions (mineral moisture content < 3%, relative humidity (RH) ≤ 97.30%). Atrazine-desisopropyl-2-hydroxy and 2-hydroxyatrazine were obtained as the main products through the dealkylation and hydrolysis of ATZ, and the intermediates exhibited low ecotoxicity toward aquatic organisms. The experiments were conducted at 23°C (RH: 6.40-97.30%). Based on quenching experiments, Mn(IV) contributed approximately 88% to ATZ degradation by accepting electrons from Cl and N in ATZ, forming Mn-Cl-C and Mn-N-H bonds, and reducing the activation energy required for hydrolysis based on DFT calculation. The oxygen vacancy in δ-MnO2 provided no considerable contribution to ATZ removal. RH notably influenced the processes, and the optimal RH value was 22.5%. Similar mechanisms were observed for simazine, simetone, propazine, and ametryn on δ-MnO2 surfaces. In agricultural soil featuring low Mn contents, ATZ degradation was limited (< 1%), however, the ATZ transformation efficiency increased to 31.2% and 63.0% after the addition of 1% and 5% δ-MnO2 particles. The effectiveness of δ-MnO2 was significantly correlated with the soil texture (R = 0.81-0.91, p < 0.05). These findings provide insights into the abiotic degradation mechanisms of triazine herbicides in dry soils, presenting strategies to remove triazine herbicide residues from soil.
干燥条件下阿特拉津在δ-MnO2表面的转化:水解和脱烷基
莠去津(ATZ)是玉米田常见的除草剂,锰氧化物(MnO2)是活性土壤成分。据报道,单独使用二氧化锰不能有效地去除水溶液中的ATZ。然而,本研究发现,在干燥条件下(矿物含水量<;3%,相对湿度(RH)≤97.30%。ATZ经脱烷基和水解得到主要产物阿特拉津-去异丙基-2-羟基和2-羟基阿特拉津,中间体对水生生物具有较低的生态毒性。实验温度为23℃(RH: 6.40-97.30%)。淬灭实验表明,Mn(IV)接受ATZ中Cl和N的电子,形成Mn-Cl- c和Mn-N- h键,并根据DFT计算降低水解所需的活化能,对ATZ的降解贡献约为88%。δ-MnO2中的氧空位对ATZ的去除没有太大的贡献。RH对工艺影响显著,最佳RH值为22.5%。在δ-MnO2表面上,西马辛、西美酮、丙嗪和阿米丁也有类似的作用机制。在低锰含量的农业土壤中,ATZ的降解受到限制(<;δ-MnO2的掺入率分别为1%和5%,ATZ的转化效率分别为31.2%和63.0%。δ-MnO2的有效性与土壤质地呈显著相关(R = 0.81-0.91, p <;0.05)。这些发现为三嗪类除草剂在干燥土壤中的非生物降解机制提供了深入的见解,并提出了清除土壤中三嗪类除草剂残留的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


