Development of electronically tuneable N-heterocyclic borates†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this contribution, we describe the synthesis and characterization of novel borates constructed with N-heterocyclic arene substituents. Beyond providing comparable electronic and redox properties to their fluoroarene analogues, the use of N-heterocycles allows for facile protonation of aromatic nitrogen atoms, thus providing a framework for tuneable reagents with varying Lewis basicity. The synthesized protonated borates were further examined as Brønstead–Lowry acids through pKa bracketing and evaluation of the kinetic accessibility of their protons by variable temperature NMR. Finally, we report the synthesis of onium and alkali metal salts of these borates through deprotonation by a variety of bases providing useful starting points for future applications.
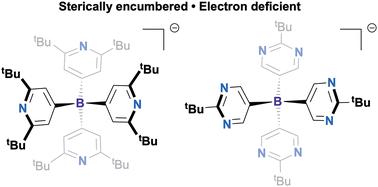
电子可调谐n -杂环硼酸盐的研制。
在这篇文章中,我们描述了用n -杂环芳烃取代基构建的新型硼酸盐的合成和表征。除了提供与氟芳烃类似物相当的电子和氧化还原性能外,n -杂环的使用允许芳香氮原子容易质子化,从而为具有不同路易斯碱度的可调试剂提供了框架。通过pKa包封和变温核磁共振评价质子的动力学可及性,进一步鉴定了合成的质子化硼酸盐为Brønstead-Lowry酸。最后,我们报道了这些硼酸盐通过各种碱的去质子化合成了铵和碱金属盐,为未来的应用提供了有用的起点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


