Effect of bath temperature on physical properties of thin films CuO using the SILAR method: Photocatalytic properties and numerical investigation
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
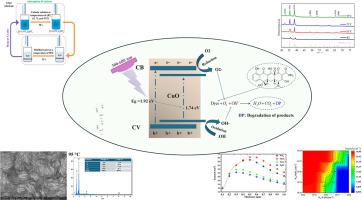
Copper oxide (CuO) thin films were deposited on glass substrates using the Successive Ionic Layer Adsorption and Reaction (SILAR) method, these films synthesized cationic solution temperatures of room temperature (RT), 65 °C, 75 °C, and 95 °C. The effects of varying cationic solution temperatures on the structural, optical, and photocatalytic properties of the CuO thin films were investigated. Characterization was performed using X-ray diffraction (XRD), UV–visible spectrophotometry (UV–Vis), and scanning electron microscopy (SEM). The XRD and SEM results revealed that all films exhibited a polycrystalline structure with monoclinic phases and good substrate coverage. The optical bandgap energy decreased from 1.92 eV to 1.74 eV as the cationic solution temperature increased. Additionally, the photocatalytic performance was evaluated by measuring the degradation of a 10 ppm tetracycline solution. The efficiencies improved from 11.1 % at RT to 18.4 % at 95 °C. Finally, a numerical analysis was conducted using the SCAPS simulation software, employing the identified optimal bandgap of 1.74 eV for degradation. The simulation involved creating a PN junction device with a CuO HTL and different electron transport layers (ETLs: ZnO, TiO2, WS2 and SnO2), to examine the effect of CuO film thickness and the shallow doping concentrations of the acceptors (CuO) and donors (ETLs) on current density.
浴液温度对CuO薄膜物理性质的影响——用SILAR方法:光催化性质和数值研究
采用连续离子层吸附和反应(SILAR)方法在玻璃衬底上沉积氧化铜(CuO)薄膜,制备的阳离子溶液温度分别为室温(RT)、65℃、75℃和95℃。研究了不同阳离子溶液温度对CuO薄膜结构、光学和光催化性能的影响。采用x射线衍射(XRD)、紫外可见分光光度法(UV-Vis)和扫描电镜(SEM)进行表征。XRD和SEM结果表明,所有薄膜均为单斜相的多晶结构,衬底覆盖率良好。随着阳离子溶液温度的升高,光学带隙能量从1.92 eV下降到1.74 eV。此外,通过测量10 ppm四环素溶液的降解来评估光催化性能。效率从室温下的11.1%提高到95℃时的18.4%。最后,利用SCAPS仿真软件进行了数值分析,选择了1.74 eV的最优带隙进行降解。该模拟涉及创建一个具有CuO HTL和不同电子传输层(ETLs: ZnO, TiO2, WS2和SnO2)的PN结器件,以研究CuO膜厚度以及受体(CuO)和供体(ETLs)的浅掺杂浓度对电流密度的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


