Prognostic significance of PD-L1 and CD45RO+ cells in glioblastoma: The modulating role of MMR status
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Background
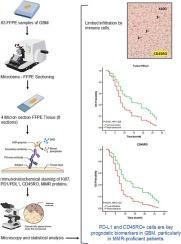
Glioblastoma (GBM) is the most prevalent and aggressive primary malignant brain tumor with limited treatment options and poor prognosis. Prognostic biomarkers, including immune checkpoint molecules and tumor-infiltrating lymphocytes, offer valuable insights into disease outcomes and potential therapeutic targets. This study uses Immunohistochemical analyses to explore the roles of PD-L1, CD45RO+ cells, and other key biomarkers in GBM prognosis.
Methods
Immunohistochemical (IHC) staining of PD-1, PD-L1, CD45RO, mismatch repair (MMR) proteins, and Ki-67 was conducted on 63 formalin-fixed paraffin-embedded (FFPE) GBM tissue samples. Statistical analyses, including Cox regression, assessed the prognostic impact of these biomarkers.
Results
IHC staining results indicated limited immune infiltration in the tumor tissue. High PD-L1 expression (HR: 1.926) and elevated CD45RO+ cells infiltration (HR: 2.122) were significantly associated with reduced OS in GBM patients. Subgroup analyses revealed that PD-L1 and CD45RO+ cells had a stronger prognostic impact in MMR-proficient patients, whereas IDH mutation status remained the only independent prognostic marker in MMR-deficient GBM cases.
Conclusions
PD-L1 and CD45RO+ cells serve as key prognostic biomarkers in GBM, particularly in MMR-proficient patients. These findings underscore the potential for personalized immunotherapies targeting immune checkpoint pathways to improve GBM outcomes. Further research is warranted to explore the therapeutic implications of these markers in GBM subgroups.
PD-L1和CD45RO+细胞在胶质母细胞瘤中的预后意义:MMR状态的调节作用
胶质母细胞瘤(GBM)是最常见和侵袭性的原发性恶性脑肿瘤,治疗方案有限,预后差。预后生物标志物,包括免疫检查点分子和肿瘤浸润淋巴细胞,为疾病结局和潜在治疗靶点提供了有价值的见解。本研究通过免疫组化分析,探讨PD-L1、CD45RO+细胞等关键生物标志物在GBM预后中的作用。方法对63例福尔马林固定石蜡包埋(FFPE) GBM组织进行PD-1、PD-L1、CD45RO、错配修复(MMR)蛋白和Ki-67的免疫组化(IHC)染色。统计分析,包括Cox回归,评估了这些生物标志物对预后的影响。结果hc染色显示肿瘤组织免疫浸润有限。高PD-L1表达(HR: 1.926)和高CD45RO+细胞浸润(HR: 2.122)与GBM患者OS降低显著相关。亚组分析显示,PD-L1和CD45RO+细胞对mmr熟练患者的预后影响更大,而IDH突变状态仍然是mmr缺陷GBM病例中唯一独立的预后标志物。结论spd - l1和CD45RO+细胞是GBM预后的关键生物标志物,特别是在mmr熟练患者中。这些发现强调了针对免疫检查点途径的个性化免疫疗法改善GBM预后的潜力。进一步的研究需要探索这些标记物在GBM亚组中的治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


