Selective monophosphorylation of cyclic diols and polyols via hemiboronic acid catalysis†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The phosphorylation of organic molecules is a biologically essential chemical transformation. Consequently, there is high demand for methods that allow for the direct, selective, and catalytic monophosphorylation of diols and complex polyols. Due to their ability to form reversible covalent bonds with hydroxy (–OH) groups, hemiboronic acids present the unique capacity to catalytically activate diols in a nucleophilic manner. Herein, we disclose a hemiboronic acid-catalyzed monophosphorylation protocol, amenable to a variety of acyclic and cyclic diols, along with the site-selective functionalization of polyols including saccharides. Mechanistic analyses comprising of kinetic experiments and computational investigation were performed to probe for the origin of the observed site-selectivities. We propose that the observed site-selectivity originates from a difference in calculated nucleophilicity between the diol oxygens in the lower energy epimer of the reactive complex, which also exhibits a kinetically stabilizing hydrogen bonding effect with the approaching electrophile.
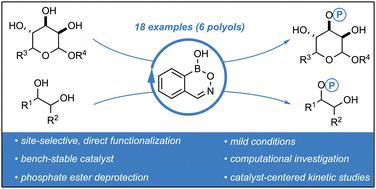
半硼酸催化环二醇和多元醇的选择性单磷酸化。
有机分子的磷酸化是生物学上必不可少的化学转化。因此,对二元醇和复杂多元醇的直接、选择性和催化单磷酸化的方法有很高的需求。由于它们与羟基(-OH)形成可逆共价键的能力,半硼酸呈现出以亲核方式催化激活二醇的独特能力。在此,我们公开了一种半硼酸催化的单磷酸化方案,适用于各种无环和环二醇,以及包括糖在内的多元醇的位点选择性功能化。力学分析包括动力学实验和计算研究,以探讨观察到的位点选择性的起源。我们认为,观察到的位点选择性源于反应配合物低能外映体中二醇氧之间计算的亲核性差异,这也表现出与接近的亲电试剂的动力学稳定氢键效应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


