A Randomized Trial of Nutrition and Exercise Treatment in Patients With Pancreatic and Non-Small Cell Lung Cancer (NEXTAC-TWO)
Abstract
Background
In our previous study (NEXTAC-ONE), the Nutrition and Exercise Treatment for Advanced Cancer (NEXTAC) program (including home-based exercise and branched-chain amino acid-containing supplements combined with nutritional counselling) was shown to potentially prevent low muscle mass-related disability in elderly cancer patients. This randomized controlled trial (NEXTAC-TWO) was conducted to elucidate whether the NEXTAC program prolongs disability-free survival in elderly patients with advanced pancreatic or non-small cell lung cancer.
Methods
This open-label, multicentre, randomized phase II study was conducted at 15 Japanese hospitals. Patients aged ≥ 70 years, with pathologically proven advanced pancreatic or non-small cell lung cancer, who were scheduled to undergo systemic chemotherapy for treatment-naïve tumours were randomly assigned (1:1) to undergo observation or receive the NEXTAC program for 12 weeks. Randomization was performed by the minimization method, using performance status and types with cancer diagnosis and anticancer treatment as adjustment factors. The primary endpoint was disability-free survival (period from randomization to the date patients were evaluated as needing care or death due to any cause). Key secondary endpoints were change in weight, muscle mass, physical activity, nutritional assessment, safety and survival. This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000028801).
Results
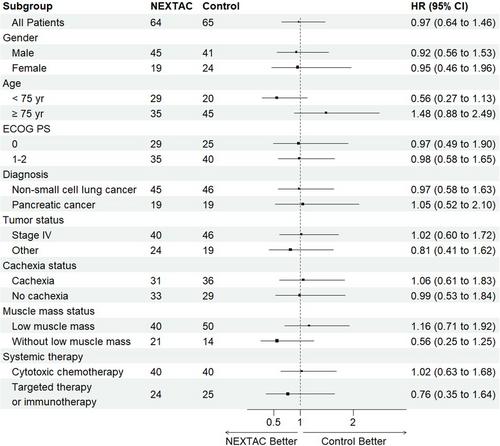
From 2017 to 2019, 131 patients were enrolled and randomly assigned to NEXTAC (n = 66) or control arms (n = 65, median age 76.0 years). After randomization, two patients in the NEXTAC arm declined further participation. As a result, 64 patients (median age 75.5 years) received at least one session of the NEXTAC program. The completion rate of the planned exercise and nutrition consultation sessions was 98.4% in the NEXTAC arm. Of the 129 patients, 91 (71%) had a disability (44 in the NEXTAC arm; 47 in the control arm). In the primary analysis, median disability-free survival periods were 478 days (95% confidence interval [CI], 358–576) in the NEXTAC arm and 499 days in the control arm (95% CI, 363–604), with no significant differences between them (p = 0.884). The hazard ratio for disability-free survival in the NEXTAC arm compared with the control arm was 0.970 (95% CI 0.642–1.465). There were no differences in the secondary endpoints between the two arms.
Conclusions
The patients had good compliance with the 12-week NEXTAC program but failed to show significant improvements in disability-free survival as compared to observation alone. Further study on the progression of low muscle mass in the NEXTAC arm is needed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


