Recovery of lithium from spent LiFexMn1-xPO4 batteries through a leaching-precipitation method using H2SO4-H2O2-Na2S2O8
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
As high-performance cathode materials, LiFexMn1−xPO4 (LMFP) batteries will become important lithium resources in the foreseeable future. In this study, a novel asynchronous oxidation approach was proposed to selectively recover Li from spent LMFP cathode powders. The spent LMFP powders were first selectively leached with H2O2 in sulfuric acid media, and the leachate was oxidatively precipitated with Na2S2O8, resulting in a Li-rich solution. During this process, Fe and Mn can also be recovered in the form of FePO4·2H2O and MnPO4·H2O, respectively. The effects of the reaction conditions on the reaction efficiency were investigated in detail. The mechanism of lithium selective extraction was studied using thermodynamics and analyses based on X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM-EDS). The total leaching efficiencies of Li, Fe, and Mn were 93.2 %, 99.9 %, and 97.0 %, respectively, under optimal conditions. A closed-loop process was finally proposed for recycling spent LMFP cathode powders. The process is environmentally friendly and economically feasible based on the technoeconomic analysis.
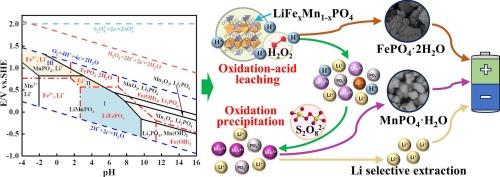
用H2SO4-H2O2-Na2S2O8浸出-沉淀法回收废LiFexMn1-xPO4电池中的锂
LiFexMn1−xPO4 (LMFP)电池作为高性能正极材料,在可预见的未来将成为重要的锂资源。在本研究中,提出了一种新的异步氧化方法来选择性地从废LMFP阴极粉末中回收锂。首先在硫酸介质中用H2O2选择性浸出LMFP粉末,然后用Na2S2O8氧化沉淀浸出液,得到富锂溶液。在此过程中,Fe和Mn也可以分别以FePO4·2H2O和MnPO4·H2O的形式被回收。研究了反应条件对反应效率的影响。利用热力学和x射线衍射(XRD)、x射线光电子能谱(XPS)、扫描电子显微镜(SEM-EDS)等分析手段研究了锂的选择性萃取机理。在最优条件下,Li、Fe和Mn的总浸出效率分别为93.2%、99.9%和97.0%。最后提出了一种闭环回收废旧LMFP阴极粉末的方法。技术经济分析表明,该工艺环境友好,经济可行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


