Probe design for high sensitivity proton-detected solid-state NMR
IF 1.9
3区 化学
Q3 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
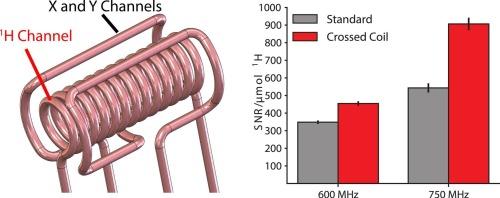
Proton (1H) detection methodologies in solid-state NMR (SSNMR) have revolutionized the field allowing for probing of new frontiers in determining the structure and dynamics within biological systems and materials. While approaches that maximally leverage the high gyromagnetic ratio of 1H detection have enhanced sensitivity and resolution of SSNMR experiments, the radiofrequency (rf) circuit of magic-angle spinning (MAS) probes is not well optimized for 1H detection, limiting the overall signal-to-noise ratio (SNR). Rather, SSNMR probes have historically been optimized for lower gamma nuclei such as 13C and below. Here we present a design with an inner coil for proton (1H) to maximize 1H sensitivity. Optimizing the 1H channel resulted in a 1.33–2-fold increase in SNR with 1H detection in a one-dimensional experiment. An outer coil is tuned to the 13C and 15N frequencies, with excellent B1 homogeneity on all three channels. Using this design, we find that the sensitivity scales better than the theoretical expectations from 600 MHz to 750 MHz, due to a combination of the improved rf efficiency and B1 homogeneity. We also demonstrate these improvements on a model protein system (GB1) with a 4D experiment collected in less than a day.
高灵敏度质子探测固态核磁共振探针设计
固态核磁共振(SSNMR)中的质子(1H)检测方法已经彻底改变了该领域,允许在确定生物系统和材料的结构和动力学方面探索新的前沿。虽然最大限度地利用1H检测的高回旋磁比的方法提高了SSNMR实验的灵敏度和分辨率,但魔角旋转(MAS)探头的射频(rf)电路并未很好地优化用于1H检测,限制了整体信噪比(SNR)。相反,ssmr探测器历来针对低γ核(如13C及以下)进行了优化。在这里,我们提出了一个内部线圈的质子(1H)的设计,以最大限度地提高1H的灵敏度。在一维实验中,优化1H通道使1H检测的信噪比提高1.33 - 2倍。外线圈调谐到13C和15N频率,在所有三个频道上都具有出色的B1均匀性。使用这种设计,我们发现在600 MHz到750 MHz范围内,由于改进的射频效率和B1均匀性的结合,灵敏度的尺度优于理论预期。我们还在不到一天的时间内收集了4D实验,在模型蛋白质系统(GB1)上展示了这些改进。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.80
自引率
13.60%
发文量
150
审稿时长
69 days
期刊介绍:
The Journal of Magnetic Resonance presents original technical and scientific papers in all aspects of magnetic resonance, including nuclear magnetic resonance spectroscopy (NMR) of solids and liquids, electron spin/paramagnetic resonance (EPR), in vivo magnetic resonance imaging (MRI) and spectroscopy (MRS), nuclear quadrupole resonance (NQR) and magnetic resonance phenomena at nearly zero fields or in combination with optics. The Journal''s main aims include deepening the physical principles underlying all these spectroscopies, publishing significant theoretical and experimental results leading to spectral and spatial progress in these areas, and opening new MR-based applications in chemistry, biology and medicine. The Journal also seeks descriptions of novel apparatuses, new experimental protocols, and new procedures of data analysis and interpretation - including computational and quantum-mechanical methods - capable of advancing MR spectroscopy and imaging.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


