Dual Photoredox/Cu-Catalysis Enables Diastereoselective Synthesis of 3-β-Ethynyltropanes via Decarboxylative Alkynylation
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
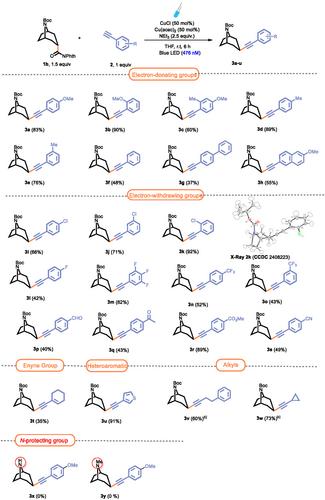
An efficient decarboxylative alkynylation of a tropane redox-active ester with terminal alkynes is described, yielding ethynyltropanes with exclusive β-configuration. This cost-effective process utilizes mild, nontoxic copper-catalyzed, photoinduced reaction conditions. Beyond introducing a novel pathway for the catalytic alkynylation of nortropanes, this method offers a versatile and convergent approach for synthesizing complex nortropane derivatives through further functionalization of the triple bond, including reductions, and inverse demand Diels–Alder reactions.


双光氧化还原/Cu催化通过脱羧烷基化实现了3 - β -乙基tropanes的非对映选择性合成
描述了一种有效的脱羧烷基化反应,将一种tropane氧化还原活性酯与末端炔进行烷基化反应,得到具有独特β构型的乙基tropane。这种低成本高效的工艺利用温和、无毒的铜催化光诱导反应条件。除了引入一种新的途径来催化炔基化nortropane外,该方法还提供了一种通用的、收敛的方法来合成复杂的nortropane衍生物,通过进一步的三键功能化,包括还原和逆需求Diels-Alder反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


