Harnessing dicarboxylate-based salts for enhancing the performance of amorphous solid dispersions: The case of olanzapine
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-11
DOI:10.1016/j.ejpb.2025.114782
引用次数: 0
Abstract
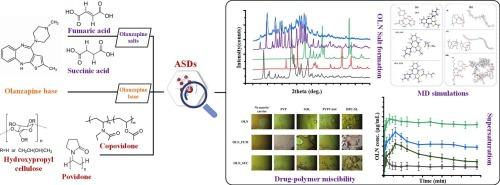
Previous studies have shown that the use of dicarboxylic acid salts can improve the performance of drug amorphous solid dispersions (ASDs), such as in the case of olanzapine (OLN). However, these studies focused only on the use of limited ASD matrices/carriers, e.g. polyvinyl alcohol, overlooking thus the critical impact of their selection on the drug’s physical stability and dissolution performance. This study evaluates the performance of ASDs containing fumarate (FUM) and succinate (SUC) drug salts in comparison to ASDs prepared with the drug base, by utilizing the same model drug (i.e., OLN) and various ASD matrices/carriers. Results revealed that inappropriate matrix/carrier selection, even with the more stable amorphous drug salts, led to physical instability (i.e., drug recrystallization) during long term storage. Specifically, only certain matrices/carriers, such as Soluplus® (SOL) for OLN_SUC, copovidone (PVPVA64) or hydroxypropyl cellulose (HPC-SL) for OLN_FUM, and povidone (PVP) for OLN base, were effective in preventing drug recrystallization after three months of storage. Molecular interactions, supported by differential scanning calorimetry (DSC), attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy and molecular dynamics (MD) simulations, demonstrated strong hydrogen bonds between OLN molecules (base and salts) and specific matrices/carriers, contributing to the system’s physical stability. Dissolution studies conducted under non-sink conditions further highlighted the importance of matrix/carrier selection for drug supersaturation, with OLN_FUM and OLN_SUC ASDs showing superior performance (higher degree of supersaturation) compared to OLN in its base form. However, all OLN_FUM formulations, as well as OLN_SUC-based ASDs with SOL, showed significant physical instability during solubilization, leading to API’s recrystallization in the precipitates collected after dissolution - a pitfall that was not observed in ASD prepared with OLN base and SOL. Hence, the obtained results highlight the necessity of extensive pre-formulation and formulation studies in the preparation of ASDs for various poorly water-soluble drugs (such as OLN) before suggesting dicarboxylate-based drug salts as a one-size-fits-all solution.
利用二羧酸盐增强无定形固体分散体的性能:以奥氮平为例。
先前的研究表明,使用二羧酸盐可以改善药物非晶固体分散体(asd)的性能,例如奥氮平(OLN)。然而,这些研究只关注有限的ASD基质/载体的使用,例如聚乙烯醇,因此忽略了它们的选择对药物的物理稳定性和溶解性能的关键影响。本研究通过使用相同的模型药物(即OLN)和不同的ASD基质/载体,对含有富马酸盐(FUM)和琥珀酸盐(SUC)药物盐的ASD与用药物碱制备的ASD的性能进行了比较。结果表明,即使使用更稳定的非晶态药物盐,不适当的基质/载体选择也会导致药物在长期储存过程中的物理不稳定性(即药物再结晶)。具体来说,只有特定的基质/载体,如用于OLN_SUC的Soluplus®(SOL),用于OLN_FUM的copovidone (PVPVA64)或羟丙基纤维素(HPC-SL),以及用于OLN碱的聚维酮(PVP),在储存三个月后才能有效防止药物重结晶。差示扫描量热法(DSC)、衰减全反射傅立叶变换红外(ATR-FTIR)光谱和分子动力学(MD)模拟支持的分子相互作用表明,OLN分子(碱和盐)与特定基质/载体之间存在强氢键,有助于系统的物理稳定性。在非沉淀条件下进行的溶出研究进一步强调了基质/载体选择对药物过饱和的重要性,与碱形式的OLN相比,OLN_FUM和OLN_SUC asd表现出更优越的性能(过饱和程度更高)。然而,所有的OLN_FUM配方,以及oln_su -based ASD与SOL在增溶过程中表现出明显的物理不稳定性,导致API在溶解后收集的沉淀物中再结晶,这一缺陷在OLN碱和SOL制备的ASD中没有观察到。所获得的结果强调了在为各种水溶性差的药物(如OLN)制备asd时进行广泛的配方前和配方研究的必要性,然后才建议以二羧酸盐为基础的药物盐作为一种通用的溶液。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


