Pulsed electrolysis: An efficient approach to enhancing purity from 4N to 6N copper
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
The conventional direct-current (DC) electrorefining process is widely used for the purification of blister copper to achieve high-purity grades. However, this process typically requires extended operational cycles and exhibits limitations in removing trace impurities such as silver and tin. To address these challenges, a pulsed electro-refining method is proposed that achieves enhanced impurity removal efficiency and shorter purification time. The experimental results showed that higher impurities removal efficiency and better appearance of high-purity copper deposits could be reached in the pulse electrolysis process. The total impurities concentration of copper products could be reduced to 0.74 mg/kg after 24 h by pulse electro-refining, which could only reach 1.24 mg/kg by DC electrolysis. Moreover, the silver and tin concentrations decreased by 95 % and 85 %, respectively. In addition, the effects of several crucial conditions during the pulsed electrolysis process were investigated, including pulse current density, pulse frequency, pulse duty ratio, and pulse electrolysis duration. The results indicated that the concentrations of all impurities apart from silicon were reduced and the purity of copper deposits reached 6 N under the experimental conditions of pulse current density of 400 A/m2, pulse frequency of 500 Hz, and pulse duty ratio of 50 % after 36 h. In summarily, the pulsed electrolysis process demonstrates excellent efficacy in producing high-purity copper.
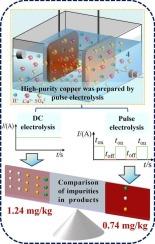
脉冲电解:一种提高从4N到6N铜纯度的有效方法
传统的直流电精炼工艺被广泛用于泡罩铜的提纯,以获得高纯度等级。然而,该工艺通常需要延长操作周期,并且在去除微量杂质(如银和锡)方面表现出局限性。为了解决这些问题,提出了一种脉冲电精炼方法,以提高杂质去除效率和缩短净化时间。实验结果表明,脉冲电解可获得较高的杂质去除率和较好的高纯铜镀层外观。脉冲电精炼24 h后铜产品总杂质浓度可降至0.74 mg/kg,而直流电解只能达到1.24 mg/kg。此外,银和锡的浓度分别下降了95%和85%。此外,还研究了脉冲电解过程中几个关键条件的影响,包括脉冲电流密度、脉冲频率、脉冲占空比和脉冲电解时间。结果表明,在脉冲电流密度为400 A/m2、脉冲频率为500 Hz、脉冲占空比为50%的实验条件下,脉冲电解36 h后,除硅外的杂质浓度均有所降低,铜镀层纯度达到6 N。综上所述,脉冲电解工艺在生产高纯铜方面效果良好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


