Promises and pitfalls of multi-cancer early detection using liquid biopsy tests
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Cancer screening is an essential public health intervention for diagnosing cancers at an early stage that can enable earlier treatment — ideally with curative intent — and thus lead to improved outcomes. Over the past decade, liquid biopsy-based tests have emerged as a promising, minimally invasive and broadly applicable screening approach by combining multi-cancer early detection (MCED) with tumour tissue-of-origin identification. Large-scale randomized clinical trials evaluating liquid biopsy-based MCED approaches are now under way, although whether the diagnostic performance of this first generation of MCED tests is sufficient to translate into clinical benefits remains to be determined. In this Review, we discuss the promises and pitfalls of current MCED tests and highlight possible trajectories for the field of early cancer detection. Liquid biopsy-based tests have demonstrated potential as a minimally invasive and broadly applicable approach to simultaneously screen individuals for multiple cancer types. In this Review, Wan, Sasieni and Rosenfeld discuss the promises and limitations of such multi-cancer early detection tests as well as future directions for this field.
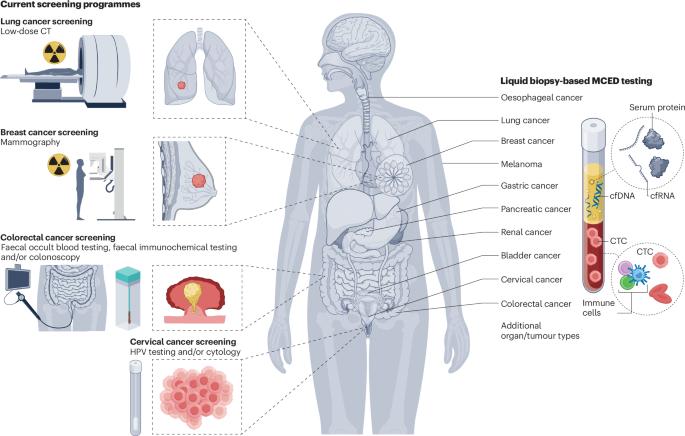

液体活检检测早期检测多种癌症的前景和缺陷
癌症筛查是在早期阶段诊断癌症的一项必要的公共卫生干预措施,可以使早期治疗成为可能——理想情况下是具有治疗目的——从而改善结果。在过去的十年中,基于液体活检的检测已经成为一种有前途的、微创的、广泛适用的筛查方法,它将多种癌症早期检测(MCED)与肿瘤起源组织识别相结合。目前正在进行大规模随机临床试验,评估基于液体活检的MCED方法,尽管第一代MCED测试的诊断性能是否足以转化为临床益处仍有待确定。在这篇综述中,我们讨论了当前MCED测试的前景和缺陷,并强调了早期癌症检测领域可能的发展轨迹。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


