Compatibility of antimicrobial preservatives with therapeutic bacteriophages of the genera Pbunavirus and Kayvirus
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-10
DOI:10.1016/j.ejpb.2025.114781
引用次数: 0
Abstract
Implementing bacteriophages into dosage forms is a significant step for the practical application of phage therapy. While designing a dosage form, bacteriophages as active ingredients may be exposed to excipients, guaranteeing microbial quality. However, only a few antimicrobial preservatives have been studied regarding their interaction with bacteriophages during long-term storage. Here, the stability of the staphylococcal Kayvirus and pseudomonal Pbunavirus with twelve commonly used preservatives was monitored for thirteen weeks to assess the risk of destabilisation of phage suspensions by excipients. The effectiveness of preservatives on the test bacteria, yeast and mould was determined using a microdilution method and the phage lytic activity by plaque enumeration. The antimicrobial activity of preservatives with bacteriophages was confirmed, except benzalkonium chloride and chlorhexidine digluconate, which showed precipitation and were classified as incompatible. A complete loss of phage potency in both tested phages occurred with diazolidinyl urea and in Kayvirus with benzalkonium chloride. For both phages, a slight decrease in titer, by one order of magnitude, was observed with m-cresol, sodium propionate, sodium benzoate, and phenylethyl alcohol. For Kayvirus, thimerosal, parabens, and mono propylene glycol and for Pbunavirus, phenoxyethanol also met the criteria. The decrease by two or more orders was determined for the remaining cases. This study helps select antimicrobial preservatives for optimizing dosage formulations with the therapeutically applicable bacteriophages.
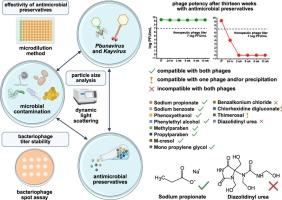
抗菌防腐剂与Pbunavirus和Kayvirus属治疗性噬菌体的相容性
将噬菌体制成剂型是噬菌体治疗实际应用的重要一步。在设计剂型时,噬菌体作为活性成分可与辅料接触,保证微生物质量。然而,只有少数抗菌防腐剂在长期储存过程中与噬菌体的相互作用被研究过。在这里,使用12种常用的防腐剂对葡萄球菌Kayvirus和假单胞Pbunavirus的稳定性进行了为期13周的监测,以评估辅料对噬菌体悬浮液不稳定的风险。用微量稀释法测定防腐剂对试验菌、酵母菌和霉菌的作用,用菌斑计数法测定噬菌体裂解活性。除苯扎氯铵和二光酸氯己定表现出沉淀,被归为不相容外,其他防腐剂与噬菌体均有抗菌活性。在两种被测噬菌体中,重唑烷酰脲和苯扎氯铵均使噬菌体效力完全丧失。对于这两种噬菌体,观察到间甲酚、丙酸钠、苯甲酸钠和苯乙醇的滴度略有下降,下降了一个数量级。对于Kayvirus、硫柳汞、对羟基苯甲酸酯和单丙二醇以及Pbunavirus,苯氧乙醇也符合标准。减少两个或更多的订单被确定为剩余的情况。本研究有助于选择抗菌防腐剂,以优化治疗适用噬菌体的剂量配方。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


